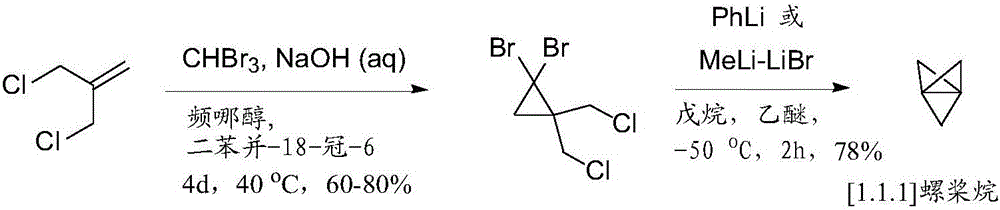
Propellane derivates and synthesis
A technology of propane and alkyl, applied in the field of synthetic organic chemistry, which can solve the problems of reagent availability and cost constraints for commercial use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Example 1: General procedure
[0137] Prepare anhydrous EtOH, anhydrous MeOH, or anhydrous MeOH and anhydrous Et containing 1 ppm BHT (10 mM final concentration) 2 Fe(acac) in a ~2:1 mixture of O 3 solution (30 mol% or 50 mol%) and in N 2 under stirring for 2min. Add [1.1.1]propane (1 equiv, as dissolved in Et 2 O solution) and a suitable reagent (1.2-3 equiv.) capable of providing all or part of the substituents, followed by the addition of PhSiH 3 (1.0-1.5 equiv.). After stirring overnight at room temperature (RT), the mixture containing the product was concentrated to give the desired compound, which could be further purified by flash chromatography on silica gel, or the product was continued without isolation or purification to yield the corresponding derivative .
Embodiment 2
[0138] Example 2: Bicyclo[1.1.1]pentane-1-carbonitrile:
[0139]
[0140] Bicyclo[1.1.1]pentane-1-carbonitrile was prepared according to the general procedure using 4-methylbenzenesulfonyl cyanide (TsCN) as a suitable reagent capable of providing all or part of the substituents. 1 H NMR (400MHz, MeOH-d 4 ) δ 2.40 (s, 1H), 2.31 (s, 6H).
Embodiment 3
[0141] Example 3: Bicyclo[1.1.1]pentan-1-ylmethanamine
[0142]
[0143] By dissolving bicyclo[1.1.1]pentane-1-carbonitrile in anhydrous Et 2 Bicyclo[1.1.1]pentan-1-ylmethanamine was prepared by O (0.090M) and cooled to 0°C. A 2M solution of LAH in THF (5.3 equiv) was added dropwise with stirring. After 30 min, the solution was quenched at 0 °C by addition of EtOAc (2 mL). The mixture was concentrated to 30% of its original volume and loaded onto a Si-p-toluenesulfonic acid resin column. Rinse the column with MeOH followed by 1N NH in MeOH 3 The desired compound was eluted. The solution was concentrated to 10% of its original volume, then acidified with 4N HCl in dioxane, and concentrated to dryness to give bicyclo[1.1.1]pentan-1-ylmethanamine. 1 H NMR (400MHz, MeOH-d 4 )δ2.97(s,2H), 2.57(s,1H), 1.90(s,6H); LC / MS(APCI) m / z 98.1[C 6 H 11 N+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com