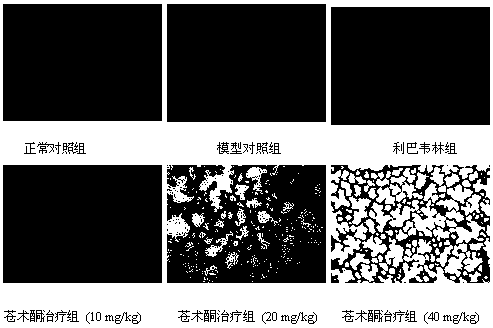
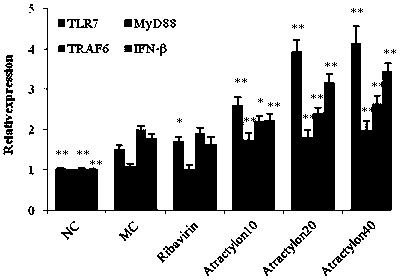
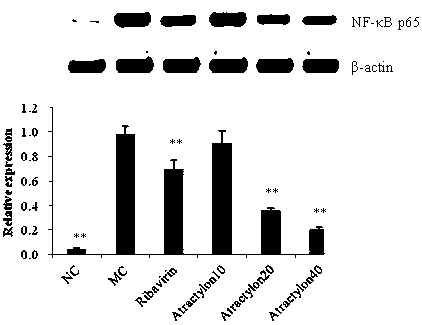
Application of atractylone in preparation of medicine for treating acute lung injury
A technology for acute lung injury and atractylodes ketone, which is applied in the field of new medical uses of atractylodes ketone, can solve the problems of no special benefit in early treatment, cannot prevent acute lung injury, etc., achieves good application prospects and application value, and relieves pulmonary inflammation. , the effect of reducing death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, supercritical carbon dioxide extraction and silica gel column chromatography separate atractylone
[0038] Supercritical carbon dioxide extraction was carried out using a HA121 supercritical liquid extraction apparatus (Huaan Instrument Factory, Jiangsu, China). Take 100 g of herb decoction pieces and crush them into powder, place them in an extraction kettle, and extract them dynamically at a pressure of 350 bar and a temperature of 50 °C for 60 min, with ethanol (99.9%) as a modifier, and a flow rate of 5 ml / min. The flow rate of supercritical carbon dioxide was set to 15g / min, and the static extraction time was set to 30min. Removal of solvent gave 5.8% product. Supercritical carbon dioxide extracts for in vitro studies were dissolved in phosphate buffer at a concentration of 1 mg / ml.
[0039] The supercritical CO2 extract (50 g) was applied to a silica gel column and separated using petroleum ether (boiling point 60~90°C) and ethyl acetate (30:1→1:1,...
Embodiment 2
[0040] The preparation of embodiment 2, atractylodes ketone tablet
[0041] Element
[0042] Add dextrin, microcrystalline cellulose, starch, lactose to Atractylodes ketone (prepared according to the method of Example 1) according to the prescription amount and mix evenly to make granules, add magnesium stearate, mix evenly, and tablet, to obtain (per Tablets containing atractylone 0.05g). The recommended oral dosage for adults is 1-4 tablets / time, 2-3 times a day.
Embodiment 3
[0043] Embodiment 3, the preparation of atractylodes capsule
[0044] Element
Dosage per capsule
atractylone
50mg
37.45mg
10.5mg
17.5mg
10.5mg
[0045] Atractylone (prepared by the method of Example 1) dextrin, microcrystalline cellulose, starch and lactose are mixed according to the prescription amount, after being made into granules, filled into No. 0 hard capsule shells, and polished to obtain (per Capsules contain atractylone 0.05g). The recommended oral dosage for adults is 1-4 capsules / time, 2-3 times a day.
[0046] The present invention will be further illustrated by the pharmacology and activity experiments and results of atractylone in the following test examples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com