Method of preparing esculentoside B through enzymatic hydrolysis of esculentoside A
A technology of pokeweed saponin A and pokeweed saponin B, applied in the field of bioengineering, can solve problems such as lack of examples, achieve high conversion efficiency, strong reaction specificity, and reduce the effect of separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
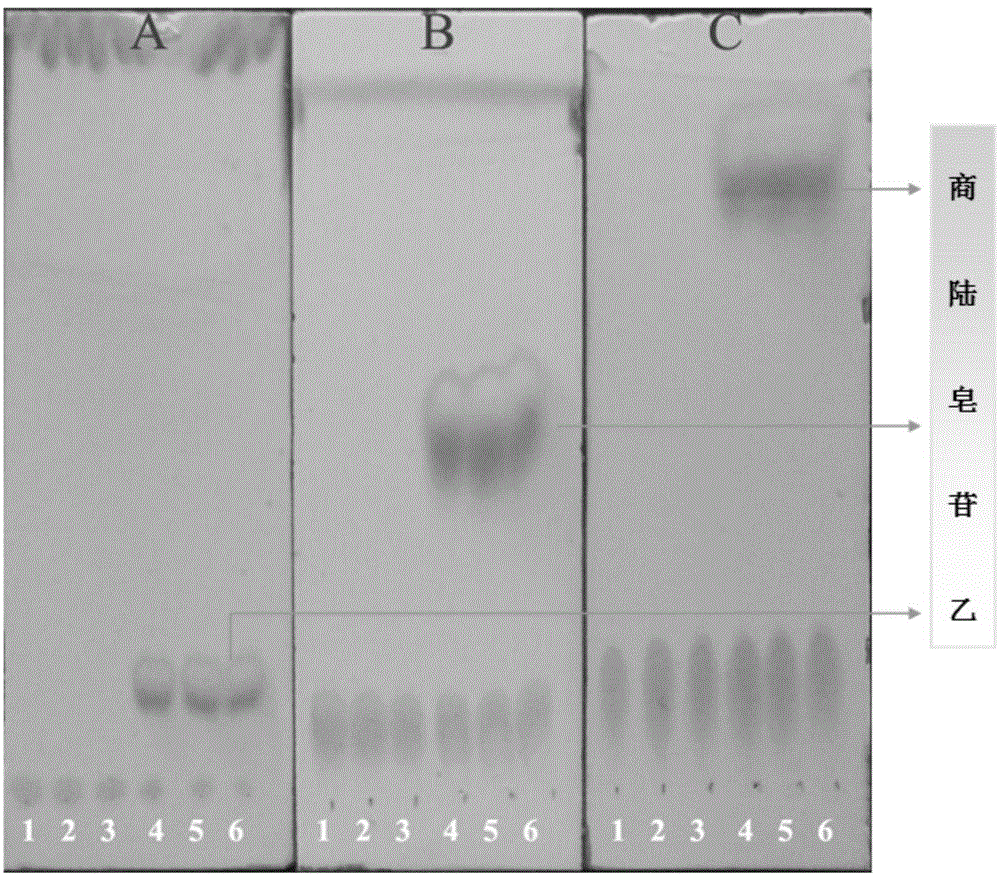
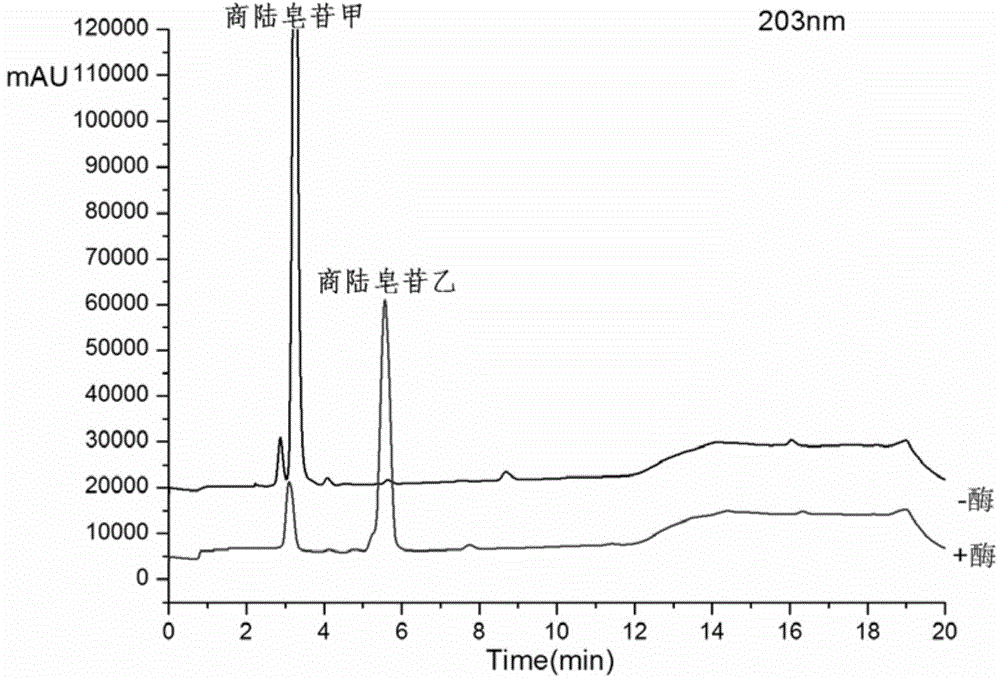
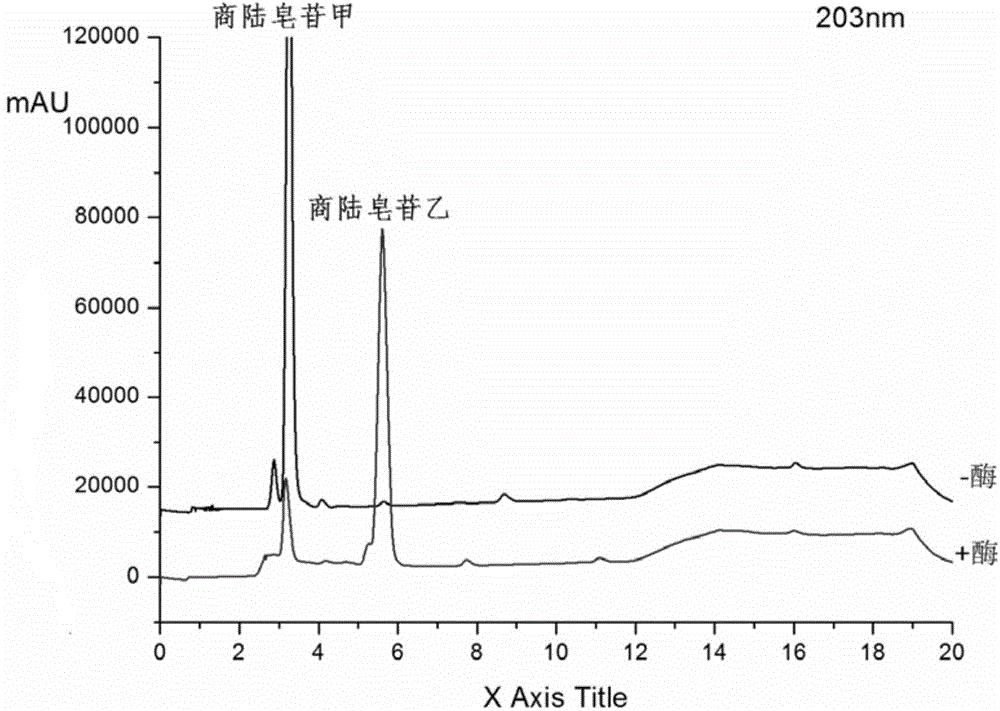
[0025] Get 100ml Erlenmeyer flask and add 11ml phosphate buffer solution (PH6.0), 1.4ml concentration is 100mg / ml beta-glucosidase solution (make the final concentration of enzyme be 10mg / ml), 1.2ml concentration is 20mM pokeweed Saponin A solution, 400ul of 20mM CaCl 2 Solution, after closing the bottle mouth, reacted at 200rpm rotary shaker at 37°C for 12h, took samples every 1h and carried out TLC (such as figure 1 As shown, it is the TLC figure of Phyloside A and its reaction supernatant under the developing agent of different proportions), or HPLC analysis (detection wavelength 203nm, mobile phase is: acetonitrile (B) / pure water (A), mobile phase The ratio of A is, 0.01-8.0min, 60%-50%; 8.0-10.0min, 50%-10%; 10.0-13.0min, 10%-10%; 13.0-13.5min, 10%-60%; 13.5 -17.0min, 60%-60%), the conversion rate of pokeweed saponin A was 82.65% in 1 hour, and the conversion rate reached above 90.13% after 12 hours of reaction. like figure 2 Shown is the conversion diagram of pokewee...
Embodiment 2
[0027] Get 100ml Erlenmeyer flask and add 9.8ml phosphate buffer solution (PH6.0) successively, 2.8ml concentration is the beta-glucosidase solution of 100mg / ml (making the final concentration of enzyme be 20mg / ml), 1.2ml concentration is the quotient of 20mM Lusaponin A solution, 400ul concentration of 20mM CaCl2 solution, after closing the bottle mouth, reacted on a rotary shaker at 200rpm at 37°C for 12h, and took samples every 1h for TLC (such as figure 1 As shown, it is the TLC figure of Phyloside A and its reaction supernatant under the developing agent of different proportions), or HPLC analysis (detection wavelength 203nm, mobile phase is: acetonitrile (B) / pure water (A), mobile phase The ratio of A is, 0.01-8.0min, 60%-50%; 8.0-10.0min, 50%-10%; 10.0-13.0min, 10%-10%; 13.0-13.5min, 10%-60%; 13.5 -17.0min, 60%-60%), the conversion rate of pokeweed saponin A was 87.89% in 1 hour, and the conversion rate reached more than 93% after 12 hours of reaction. like image 3 S...
Embodiment 3
[0029] Take 100ml Erlenmeyer flask and add 5.4ml phosphate buffer solution (PH6.0), 7ml concentration is 100mg / ml β-glucosidase solution, 1.2ml concentration is 20mM pokeweed saponin A solution, 400ul concentration is 20mM CaCl 2 Solution, after closing the bottle mouth, reacted at 200rpm rotary shaker at 37°C for 12h, took samples every 1h and carried out TLC (such as figure 1 As shown, it is the TLC figure of pokeweed saponin A and its reaction supernatant under different developing agents), or HPLC analysis (detection wavelength 203nm, mobile phase is: acetonitrile (B) / pure water (A), gradient is , 0.01-8.0min, 60%-50%A; 8.0-10.0min, 50%-10%A; 10.0-13.0min, 10%-10%A; 13.0-13.5min, 10%-60%A; 13.5 -17.0min, 60%-60%A), the conversion rate of pokeweed saponin A was 95.59% in 1 hour, and the conversion rate reached more than 98% after 12 hours of reaction. like Figure 4 Shown is the transformation diagram of pokeweed saponin A under the enzyme concentration of 50mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com