Erysipelothrix rhusiopathiae subunit vaccine, preparation method and application
A technology of Erysipelas erysipelas and subunit vaccine, which is applied in the field of vaccine preparation of livestock infectious diseases, can solve the problems of unclear mechanism, weakened virulence, and interfere with the efficacy of vaccines, so as to achieve the effect of safety and avoid invasion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Obtaining the protein sequence of spaA specific gene
[0036] Our laboratory isolated a JZ08 strain from clinically dead pigs, which was identified as Erysipelothrix rhusiopathiae by PCR. The primers in Table 1 were used to amplify, and the nucleotide sequence corresponding to the truncated protein spaA of the present invention was obtained as shown in SEQ ID NO.1.
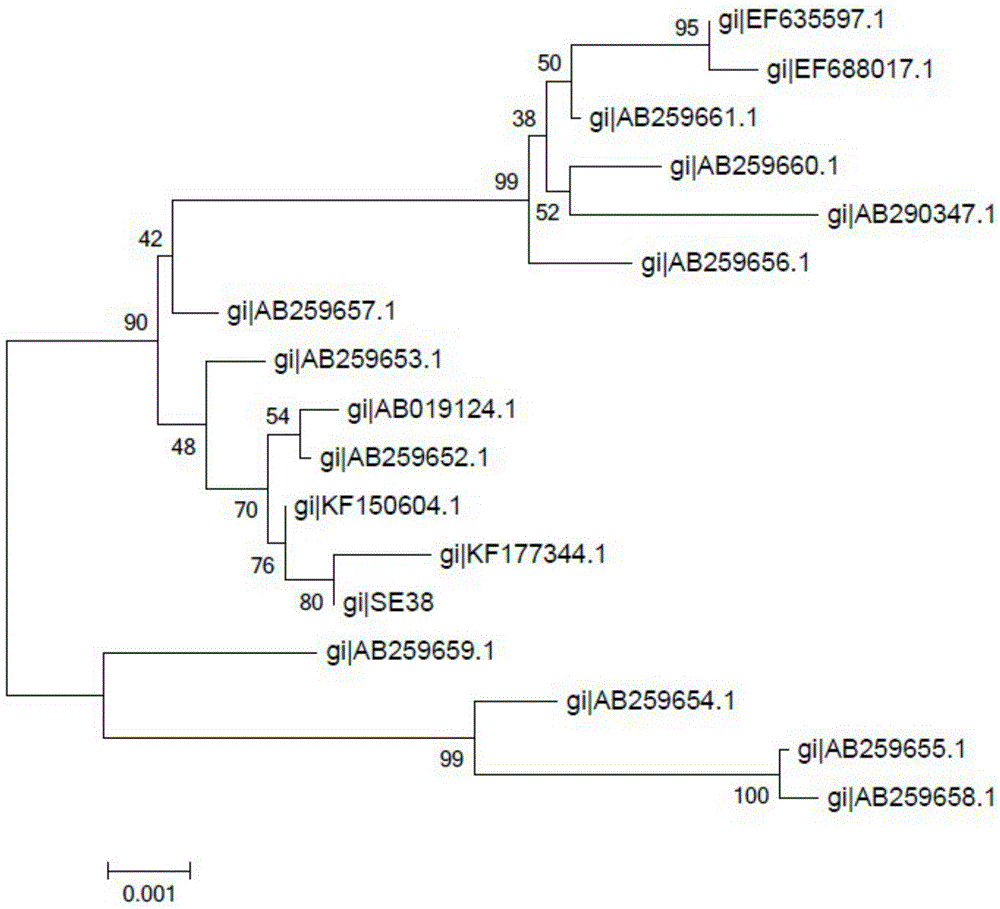
[0037] Download the full gene sequence of spaA published by NCBI and construct the phylogenetic tree. The phylogenetic tree clearly shows that the spaA sequence used in this study is different from the sequence previously published on NCBI. figure 1 .
[0038] 2) The sequence of the truncated protein spaA
[0039]The sequence of the truncated protein spaA of the present invention is shown in SEQ ID NO.2.
[0040] Build a phylogenetic tree
[0041] Phylogenetic tree analysis with the spaA protein sequence known on the Internet, it is found that the protein sequence used in this study is located on the sa...
Embodiment 2
[0043] Construction of Escherichia coli BL21 / pET28a-spaA:
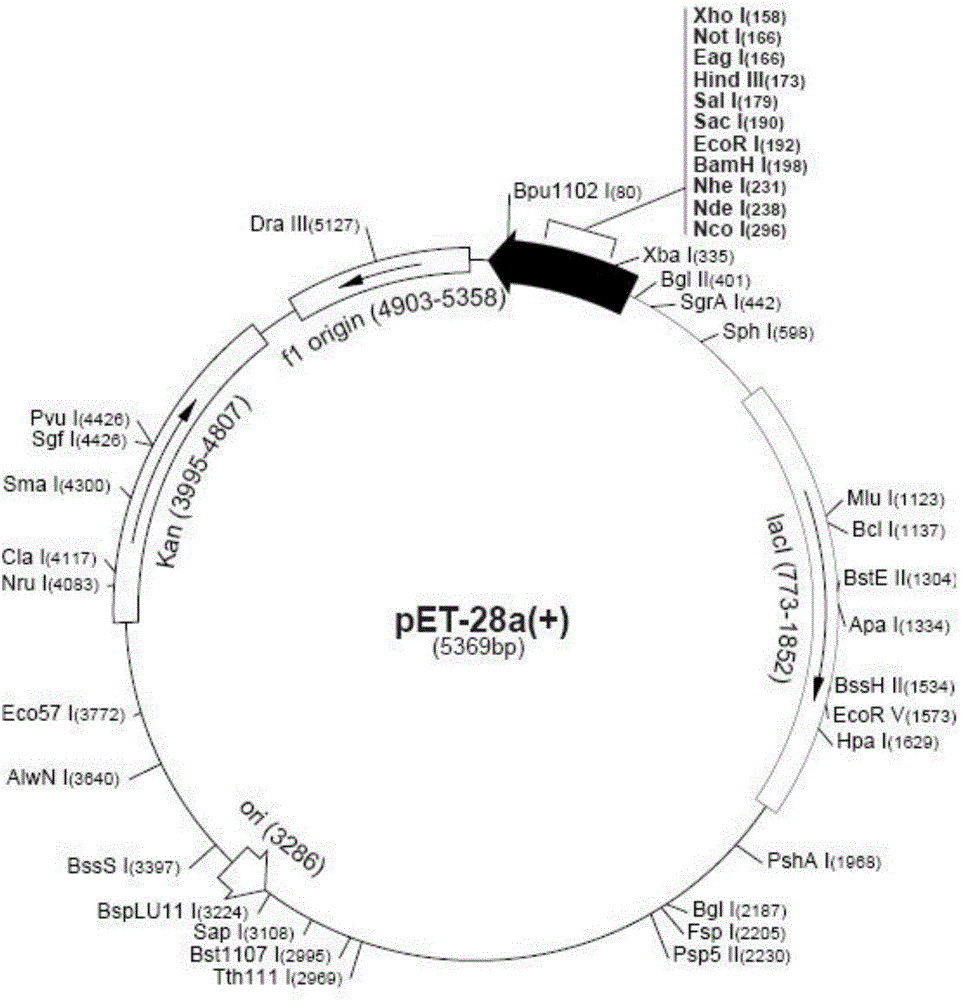
[0044] The plasmid pET-28a (+) that the present invention adopts is purchased from Noagen company (this pET-28a (+) plasmid map is as follows figure 2 Shown) Competent Escherichia coli BL21 (DE3) was purchased from Wuhan Life Technology Co., Ltd., Hubei Province, China.
[0045] Primer design and synthesis
[0046] Primer design software Primer 5.0 was used to design primers for amplifying spaA (Table 1). The primers were synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd.
[0047] Table 1: Primer design for cloning the antigenic protein gene of the present invention
[0048]
[0049] The genome of the extracted JZ08 strain was used as a PCR template. PCR amplification was performed using the primers shown in Table 1. The PCR reaction system is as follows:
[0050]
[0051]
[0052] The reaction program was: pre-denaturation at 94°C for 5 min; 94°C for 1 min; 55°C for 30 s; 72°C for 1 min...
Embodiment 3
[0064] Preparation method of recombinant protein:
[0065] 1) Escherichia coli (Escherichia coli) BL21 / pET28a-spaA was inoculated in 3 mL LB liquid medium containing 25 μg / ml Kan, and cultured on a shaker at 37°C. Take 100 μL of the cultured bacterial liquid and inoculate it into 10 mL of fresh LB liquid medium containing 25 μg / ml Kan, shake and culture at 37°C for about 3 hours, until OD 600 When it reaches 0.8, add IPTG to the final concentration of 0.8mmol / L, continue to cultivate for 3h, and then collect the bacteria.
[0066] 2) SDS-PAGE electrophoresis analysis of expression products
[0067] The induced recombinant Escherichia coli was centrifuged at 8000r / min for 15min. The pellet was resuspended with 1 / 10 volume of 50mM Tris-cl (pH 8.0), and lysozyme was added to a final concentration of 1mg / ml, and kept on ice for 30min. Ultrasonic crushing was carried out under ice bath conditions until the bacterial liquid was no longer viscous, centrifuged at 10000r / min for 30m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com