Ocular preparation for preventing and treating cataract and preparation method of ocular preparation
An ophthalmic preparation, cataract technology, applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the loss of adjustment ability, reduce the severity of cataracts, and ophthalmic preparations have not been reported. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
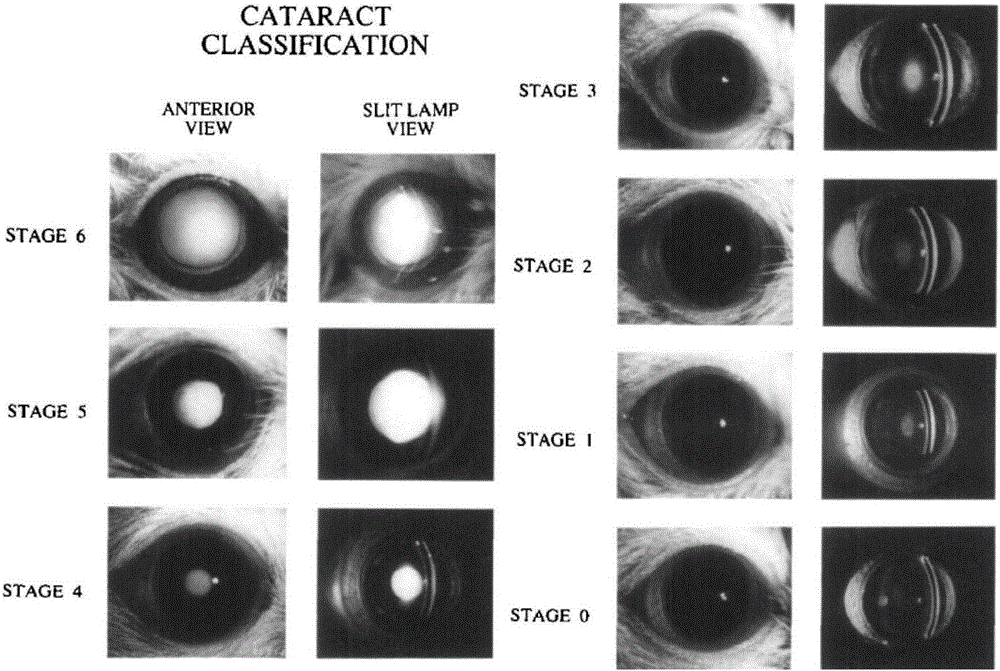
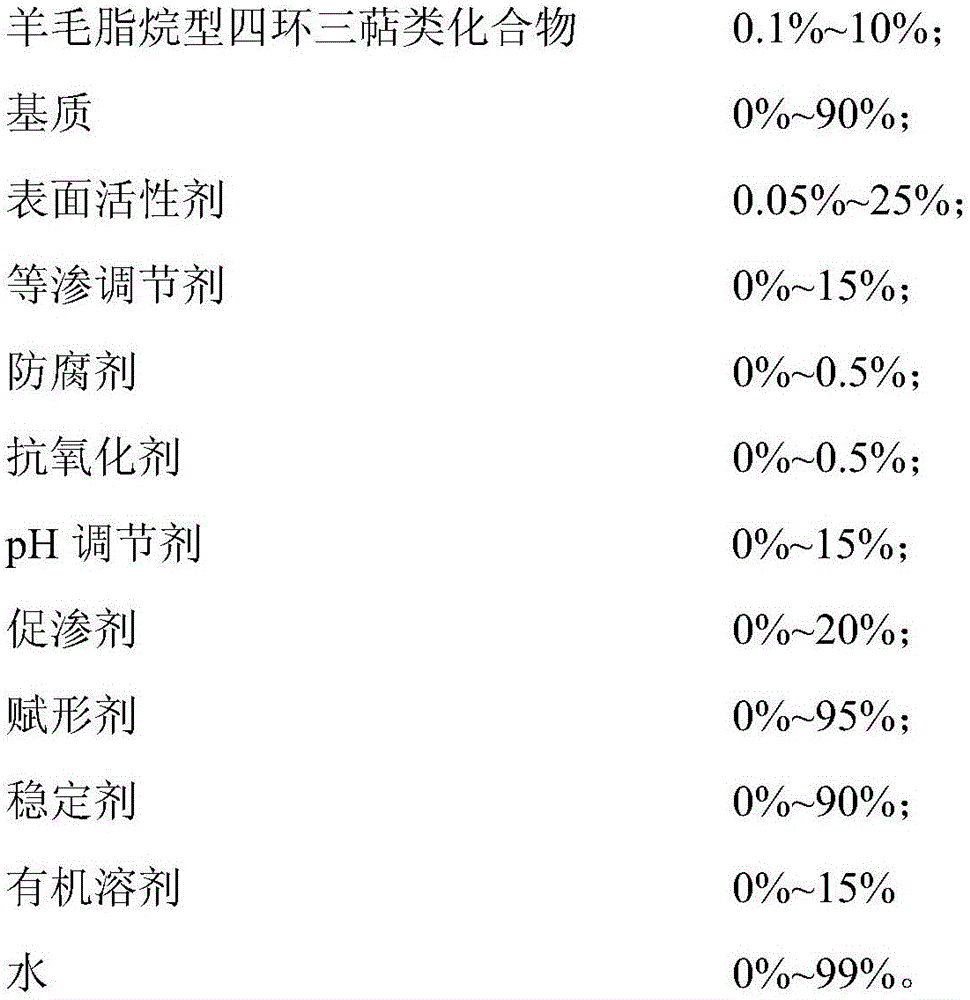
Image
Examples
Embodiment 1 to 5、 comparative example 1
[0085] Examples 1 to 5, Comparative Example 1: Refer to Table 1 for the raw material formula of lanosterol eye drops. The preparation method is as follows: weigh lanosterol and add it to a surfactant, and stir to make it fully included. Weigh the preservative and dissolve it in water for injection. Add the preservative aqueous solution to the lanosterol mixture and stir to dissolve. Autoclave, 115°C, 30min.
[0086] Table 1
[0087]
Embodiment 6 to 10、 comparative example 2
[0088] Examples 6 to 10, Comparative Example 2: Refer to Table 2 for the raw material formula of lanosterol eye ointment. The preparation method is as follows: weigh lanosterol, matrix, isotonic regulator, and some antioxidants, melt them on a water bath, and heat to 75°C. Separately weigh the surfactant, remaining antioxidant and preservative, dissolve in part of the water for injection, and heat to 75°C. Slowly add to the oil phase, stir while adding, add the remaining water for injection, and mix well.
[0089] Table 2
[0090]
[0091]
Embodiment 11 to 15、 comparative example 3
[0092] Examples 11 to 15, Comparative Example 3: Refer to Table 3 for the raw material formula of lanosterol gel. The preparation method is as follows: add the matrix to water for injection to moisten and swell, add preservative, and stir to dissolve. Add lanosterol to the surfactant and stir to dissolve. Then slowly add the lanosterol solution into the matrix solution, stir and mix evenly.
[0093] table 3
[0094]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com