Method for preparing high-purity menstrual-blood-derived stem cells
A stem cell and high-purity technology, which is applied in the field of in vitro culture expansion and cryopreservation, and the preparation of high-purity menstrual blood-derived stem cells. Clear and safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
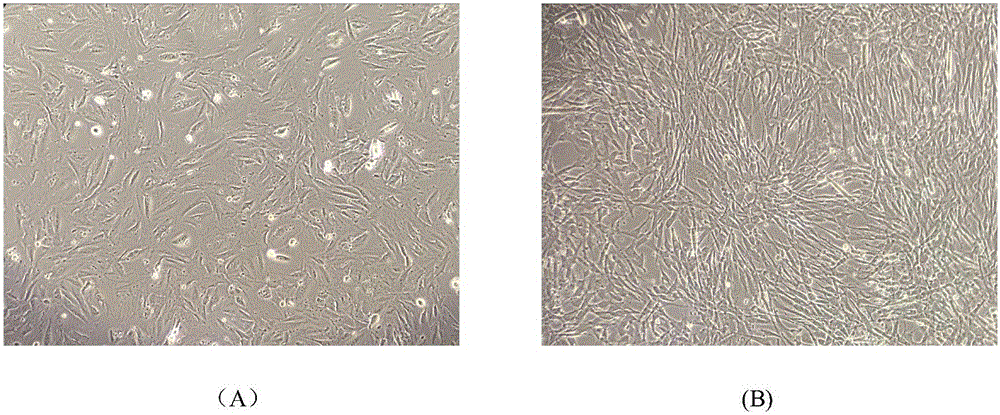
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of highly pure menstrual blood-derived stem cells
[0036] The preparation of high-purity menstrual blood-derived stem cells in this embodiment specifically includes the following steps:
[0037] (1) Use a menstrual blood collection kit to collect menstrual blood into a menstrual blood collection tube containing antibiotic preservation solution. The mixed volume ratio of antibiotic preservation solution to menstrual blood is 1:1. Store at low temperature at 2-8°C, and the storage period is ≤72 hours;
[0038] The antibiotic preservation solution is based on HBSS (Hank's Balanced Salt Solution), containing 420 units of heparin sodium, 250 μg / mL of cephalexin, 120 μg / mL of gentamicin and 3 μg / mL of amphotericin B.
[0039] (2) Centrifuge the collection tube at 600×g for 10 minutes, discard the supernatant, and collect the cell pellet.
[0040] (3) After fully mixing the cell pellet and normal saline at a volume ratio of 1:1, slowly add it to the in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com