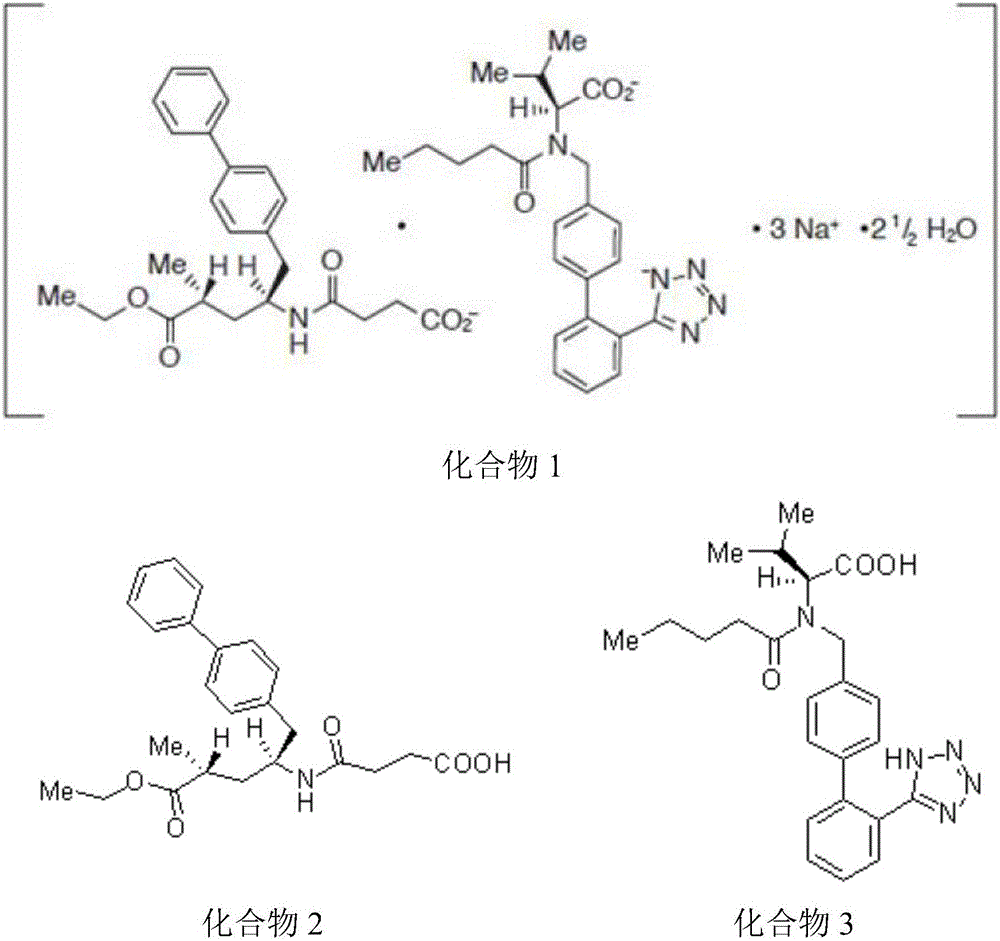
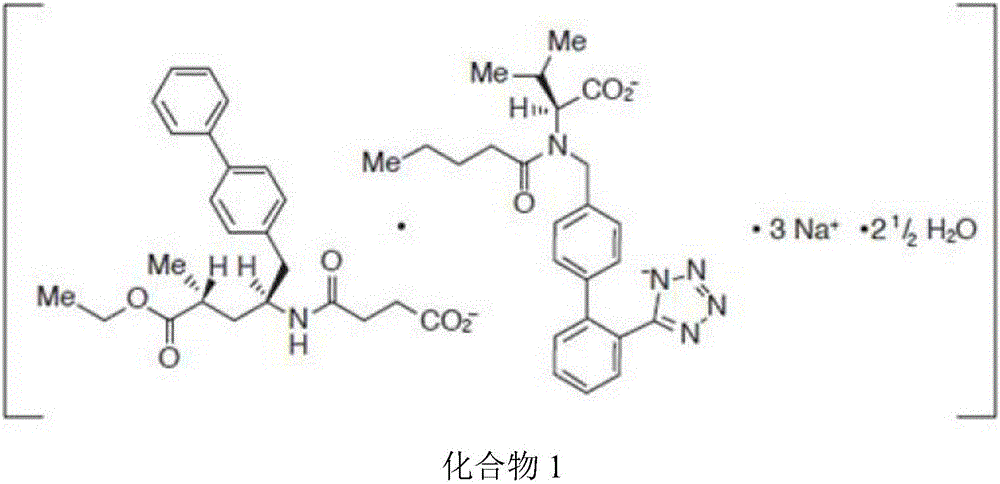
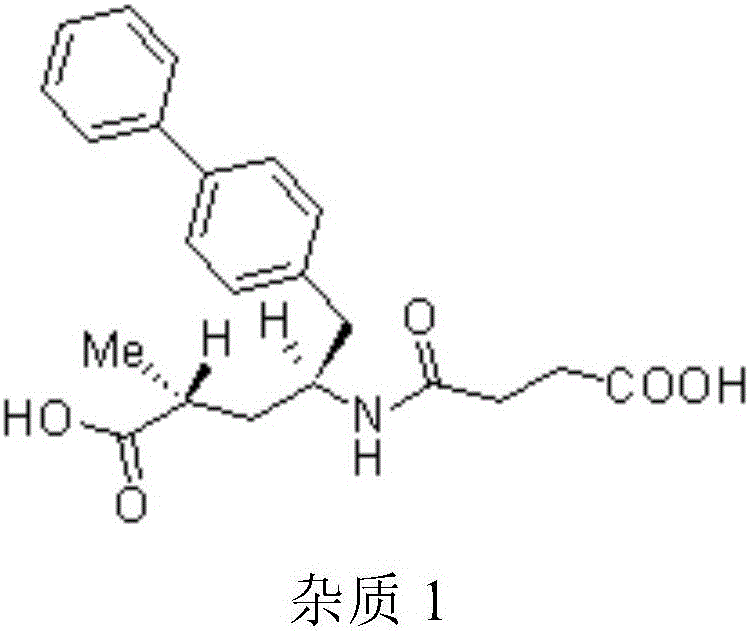
Solid oral preparation and preparation method thereof
A solid and preparation technology, which is applied in the field of pharmaceutical preparations and can solve the problems that the stability of preparations has not been studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] 1. Use a double-cone dryer to dry the moisture content of the auxiliary materials to about 1.0% (80°C);
[0050] 2. Pass microcrystalline cellulose, highly substituted hydroxypropyl cellulose, crospovidone, silicon dioxide, and magnesium stearate through a 40-mesh sieve for later use, and weigh and prepare materials according to the prescription quantity;
[0051] 3. Take the prescribed amount of crospovidone, high-substituted hydroxypropyl cellulose and 1 / 2 of the prescribed amount of microcrystalline cellulose and mix evenly to obtain mixed powder A;
[0052] 4. Take the prescribed amount of bulk drug (water content 5.0%, Karl Fischer method), silicon dioxide, and 1 / 2 of the prescribed amount of microcrystalline cellulose and mix uniformly to obtain mixed powder B;
[0053] 5. Add the mixed powder A to the mixed powder B, and add the prescribed amount of magnesium stearate for mixing to obtain the total mixed powder;
[0054] 6. The blended powder is prep...
Embodiment 2
[0058]
[0059]
[0060] The compound 1 solid oral preparation was prepared by the same method as in Example 1 by using the compound 1 bulk drug with a water content of 4.8% (Karl Fischer method).
[0061] Adopt Opadry (Opadry) coating material, use 70% hydrous ethanol as the coating solution of solvent preparation content 8% to carry out spray coating to the obtained plain tablet, control weight gain between 2%~4%, enter air The temperature is 40-60°C, and the drying time is 60 minutes.
[0062] The water content of the obtained solid oral preparation was detected by Karl Fischer method to be 5.3%.
Embodiment 3
[0064]
[0065] The compound 1 solid oral preparation was prepared by the same method as in Example 1 by using the compound 1 bulk drug with a water content of 5.1% (Karl Fischer method).
[0066] Adopt Opadry (Opadry) coating material, use 70% hydrous ethanol as the coating solution of solvent preparation content 8% to carry out spray coating to the obtained plain tablet, control weight gain between 2%~4%, enter air The temperature is 40-60°C, and the drying time is 120 minutes.
[0067] The water content of the obtained solid oral preparation was detected by Karl Fischer method to be 4.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com