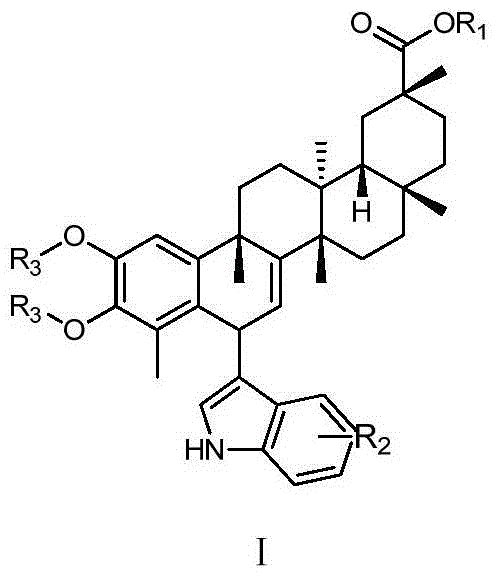
Tripterine derivative, and preparation method and use thereof
A technology of tripterine and its derivatives, which is applied in the direction of drug combinations, steroids, antineoplastic drugs, etc., can solve the problems of reduced drugability, unstable thioether bonds, weak anticancer activity, etc., and achieves reduced toxicity, Good stability and good anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Example 1 R 1 = R 2 = R 3 When =H, the preparation of compound 1
[0046] The reaction formula is as follows:
[0047]
[0048] Dissolve tripterine (1.0eq) in anhydrous dichloromethane, add indole (2.0eq) and catalyst Sc(OTf) 3 (5mol%), reacted at normal temperature for 24 hours, and TLC detected the reaction. After the reaction, the solvent was spin-dried, and the compound 1 (yield 78%, purity 99.2%) was obtained by column chromatography (petroleum ether / acetone=3 / 1) ): mp114-116°C; [α]20 D-89.78°(MeOH,c=0.2), 1 H-NMR (300MHz, CDCl 3 )δ7.87(s,1H),7.78(d,J=7.2Hz,1H),7.34(d,J=7.5Hz,1H),7.15-7.20(m,2H),6.83(s,1H), 6.32(s,1H),6.23(d,J=6.3Hz,1H),4.94(d,J=5.7Hz,1H),2.41(d,J=15.6Hz,1H),2.03-2.11(m,3H ),1.95(s,3H),1.40-1.68(m,8H),1.38(s,3H),1.31-1.23(m,2H),1.16(s,3H),1.02(s,3H),0.94- 0.89(m,1H),0.75(s,3H).HRMS(ESI-)m / z calcd for C 38 h 46 NO 6 [M+COOH] - 612.3331, found 612.3325.
Embodiment 2
[0049] Example 2 R 1 = R 3 = H, R 2 =-CH 3 , R 2 Preparation of compound 2 when replacing the H on the 7-position of the indole ring
[0050] The reaction formula is as follows:
[0051]
[0052] Except that the indole in Example 1 is replaced by 7-methylindole, all the other reaction conditions are the same as in Example 1 to obtain compound 2 (65% yield, 98.5% purity): mp92-94°C; [α ]20 D-78.39°(MeOH,c=0.2), 1 H-NMR (300MHz, CDCl 3 )7.72(s,1H),6.62(d,J=7.8Hz,1H),6.99-7.10(m,2H),6.84(s,1H),6.33(d,J=1.8Hz,1H),6.21( d,J=6.3Hz,1H),4.93(d,J=6.3Hz,1H),2.46(s,3H),2.39-2.43(dd,J=15.0Hz,0.6Hz,1H),2.03-2.06( m,3H),1.96(s,3H),1.46-1.73(m,9H),1.38(s,3H),1.16(s,3H),1.02(s,3H),1.01(s,3H),0.88 -0.93(m,1H),0.73(s,3H).HRMS(ESI)m / z calcd for C 38 h 47 NO 4 Na[M+Na] + 604.3396, found 604.3397.
Embodiment 3
[0053] Embodiment 3 Parallel reaction
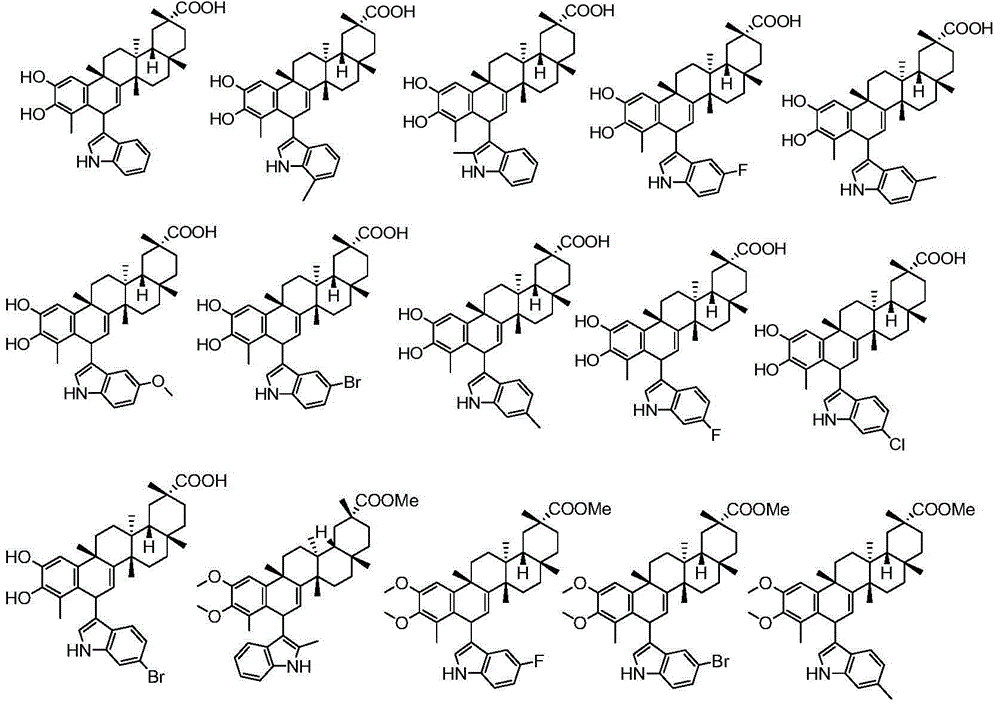
[0054] Referring to the preparation method of compound 1 in Example 1, using the corresponding substituted indole as the reaction substrate, the reaction was carried out to obtain compounds 3-11, the structural formula of which is as follows:
[0055]
[0056] Analysis data of some compounds:
[0057] Compound 4: mp96-98℃; [α]20 D-66.31°(MeOH,c=0.2), 1 H-NMR (300MHz, CDCl 3 )δ7.94(s,1H),7.87(s,1H),7.22(m,3H),6.83(s,1H),6.32(s,1H),6.14(d,J=6.2Hz,1H), 4.86(d,J=5.8Hz,1H),2.41(m,2H),2.15–1.96(m,2H),1.93(s,3H),1.80–1.41(m,8H),1.37(s,3H) ,1.31-1.22(m,2H),1.17(s,3H),1.04(s,3H),1.02(s,3H),0.98–0.87(m,1H),0.73(s,3H).HRMS(ESI )m / z calcd for C 37 h 44 FNO 4 Na[M+Na] + 608.3147, found 608.3141.
[0058] Compound 6: mp90-92℃; [α]20 D-90.36°(MeOH, c=0.2), 1 H-NMR (300MHz, CDCl 3 )δ7.85(s,1H),7.62(dd,J=8.6,5.3Hz,1H),7.06–6.78(m,3H),6.29(d,J=1.1Hz,1H),6.17(d,J =6.3Hz,1H),4.89(d,J=6.1Hz,1H),2.40(d,J=15.1Hz,2H),2.10-2.01(m,3H),1.95(s,3H),1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com