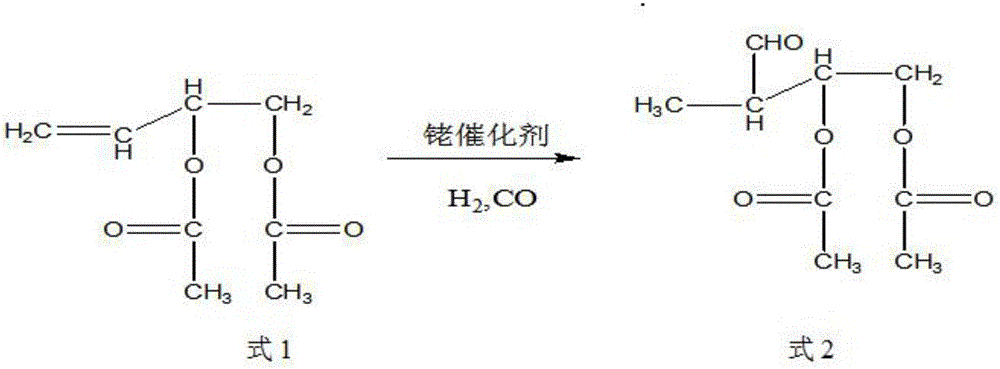
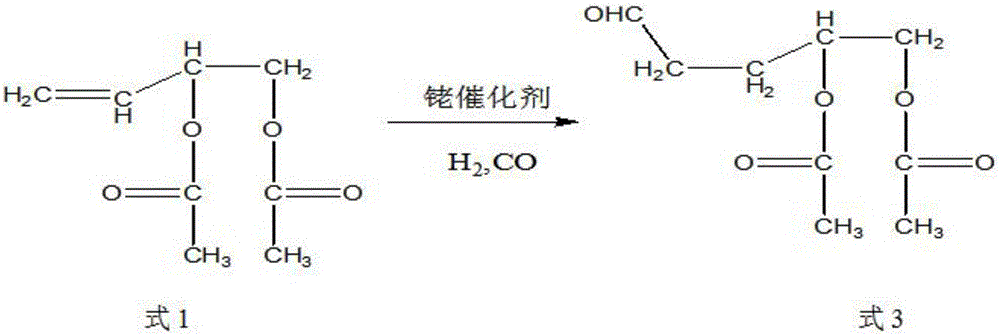
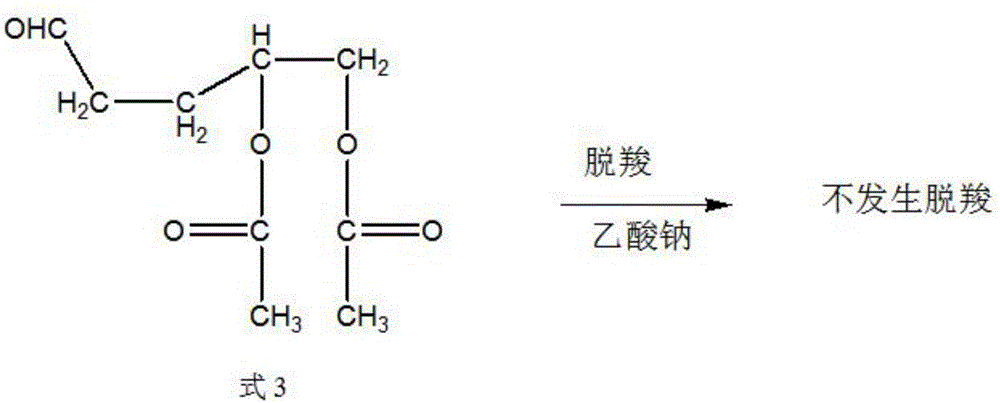
Synthetic method of trans-4-acetoxyl-2-methyl-2-butene-1-aldehyde
An acetoxy and synthetic method technology, applied in the synthesis field of trans-4-acetoxy-2-methyl-2-butene-1-aldehyde, can solve the problem of high reaction pressure requirements, low raw material utilization, The reaction requires a large pressure and other problems to achieve the effects of improving selectivity, reducing losses, and reducing production costs and process complexity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Get 100 grams of 3,4-diacetoxy-1-butene, 0.1 grams of catalyst rhodium trichloride (rhodium content 39.5%) and 9.88 grams of triphenylphosphine into the autoclave, exhaust the air, Raise the temperature to 85°C, and then use the mixed gas of hydrogen and carbon monoxide with a molar ratio of 1:2 to increase the pressure of the reactor to 100 kg, and maintain this temperature. When the pressure of the reactor drops by about 3 kg and does not continue to drop, Start to cool down to room temperature, exhaust the gas, and carry out vacuum distillation of the reaction solution to obtain 85 grams of product, the content of the target product 3-formyl-1,2-butanediol diethyl ester is 91%, and the by-product formula 3 The content is 6%.
Embodiment 2
[0033] Get 100 grams of 3,4-diacetoxy-1-butene, 0.01 grams of catalyst tris(triphenylphosphine) carbonyl rhodium hydride (rhodium content 11.2%) and 0.55 grams of triphenylphosphine together for high pressure reaction Exhaust all the air in the kettle, heat up to 100°C, then use the mixed gas of hydrogen and carbon monoxide with a molar ratio of 1:1 to raise the pressure of the reactor to 85 kg, maintain this temperature, and when the pressure of the reactor drops by 3 kg When the left and right do not continue to decline, start to cool down to room temperature, exhaust the gas, and carry out vacuum distillation of the reaction solution to obtain 93 grams of product. The content of the target product 3-formyl-1,2-butanediol diethyl ester is 95.3 %, the content of by-product formula 3 is 1.8%.
Embodiment 3
[0035] Get 100 grams of 3,4-diacetoxy-1-butene, 0.05 grams of catalyst tris(triphenylphosphine)rhodium carbonyl hydride (rhodium content 11.2%) and 1.64 grams of triphenylphosphine together for high-pressure reaction Exhaust the air, raise the temperature to 95°C, and then use the mixed gas of hydrogen and carbon monoxide with a molar ratio of 2:1 to raise the pressure of the reactor to 90 kg, maintain this temperature, and when the pressure of the reactor drops by 3 kg When the left and right do not continue to decline, start to cool down to room temperature, exhaust the gas, and carry out vacuum distillation of the reaction solution to obtain 93 grams of product. The content of the target product 3-formyl-1,2-butanediol diethyl ester is 96.3 %, the content of by-product formula 3 is 0.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com