A kind of purification method of Neratinib
A technology of neratinib and purification method, which is applied in the field of neratinib purification to achieve the effect of high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of neratinib (compound of formula 1) crude product
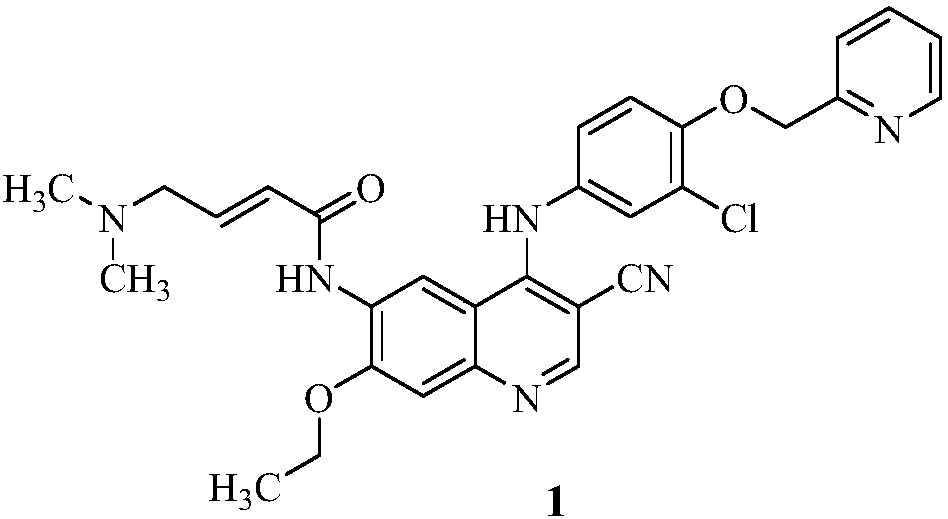
[0033] The preparation of the compound of formula I refers to the preparation method of WO2006127207, specifically as follows:
[0034]
[0035] Add 4-N,N-dimethylaminocroton hydrochloride (72g, 0.44mol), tetrahydrofuran (800ml) and dimethylformamide (0.8ml) into a 2L three-necked flask, cool to 0-5°C , Oxalyl chloride (36ml, 0.42mol) was added dropwise to the reaction solution. Then the temperature was raised to 25-30°C and stirred for 2-3 hours. The reaction solution was cooled to 0-5° C. again, and a solution (800 ml) of compound 3 (100 g, 0.22 mol) in N-methylpyrrolidone (NMP) was added dropwise. After the drop was completed, it was stirred overnight at room temperature. The reaction solution was transferred to a 10L reaction kettle, cooled to 0-5°C, quenched with water (500ml), and kept stirring at 0-5°C for 30min. Then the temperature was raised to 40° C., and 1 mol / L sodium hydr...
Embodiment 2
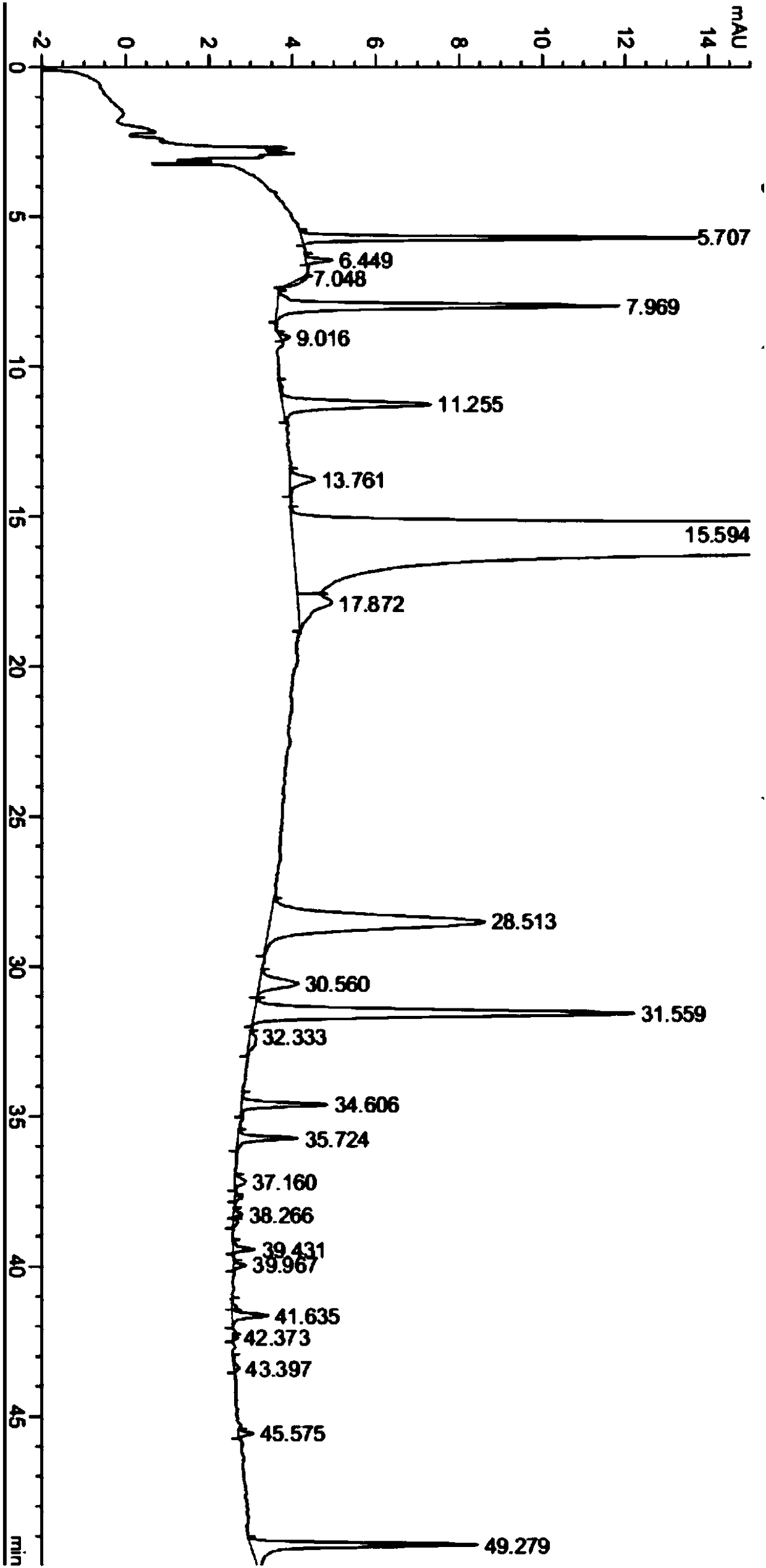
[0037] Take 10.0g of crude neratinib and add it to a 250ml round bottom flask, add 100ml dimethyl sulfoxide and 50ml tetrahydrofuran, stir and suspend, heat to 60°C, stir for 1.5 hours, then cool the suspension to 20-30°C, and stirred at 20-30°C for 1 hour, filtered through a Buchner funnel under reduced pressure, and washed the filter cake with 300ml of purified water. The obtained solid was vacuum-dried to dryness at 50-60°C to obtain 9.3g of neratinib finished product, with a purity of 99.8%. The content of simple impurities is shown in Table 2, attached figure 2 .
Embodiment 3
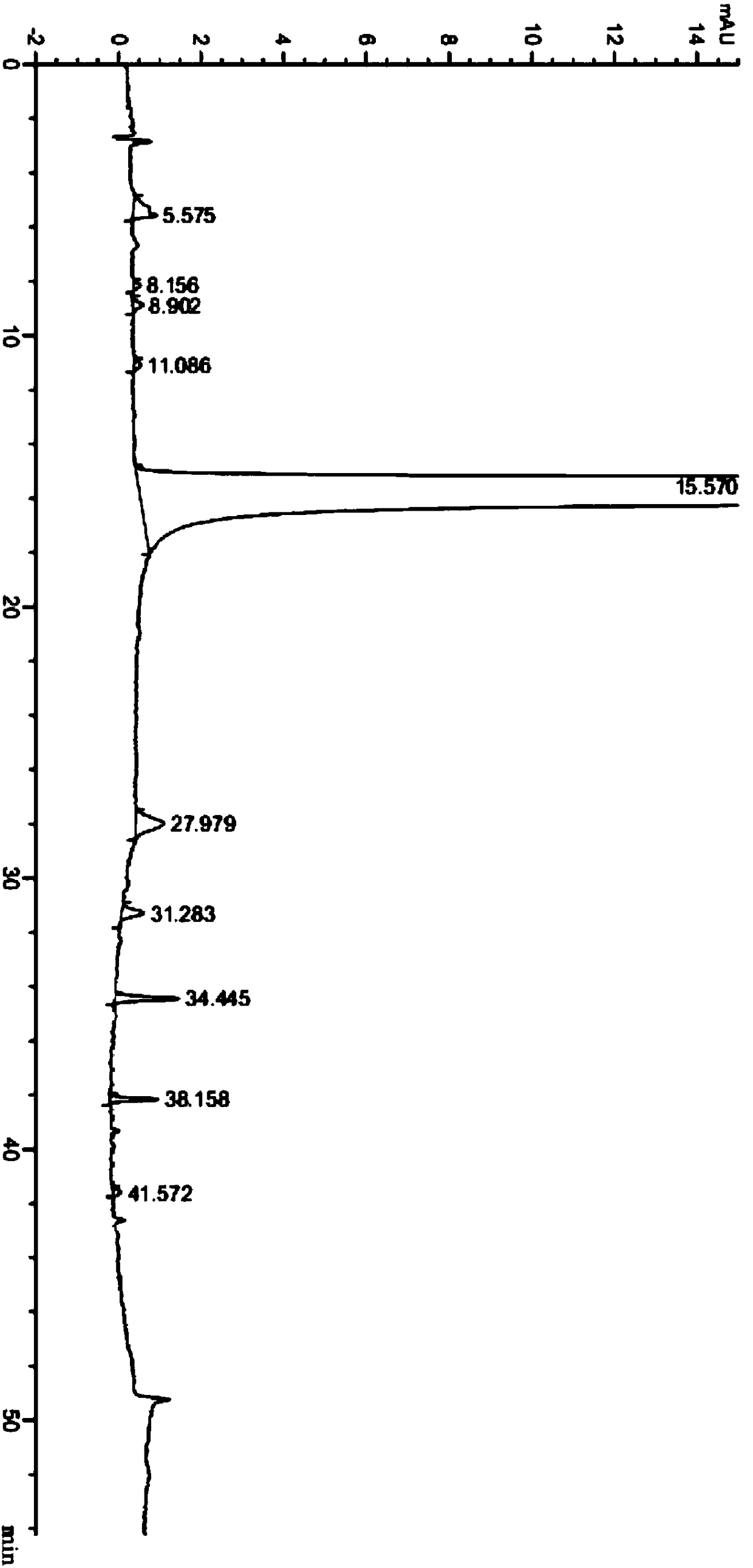
[0039] Take 10.0g neratinib crude product and add it to a 250ml round bottom flask, add 100ml dimethyl sulfoxide and 100ml tetrahydrofuran, stir and suspend, heat to 65°C, stir for 1.5 hours, then cool the suspension to 20-30°C, and stirred at 20-30°C for 5 hours, filtered through a Buchner funnel under reduced pressure, and washed the filter cake with 300ml of purified water. The obtained solid was vacuum-dried to dryness at 50-60° C. to obtain 7.8 g of neratinib as a finished product, with a purity of 99.8%. The content of simple impurities is shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com