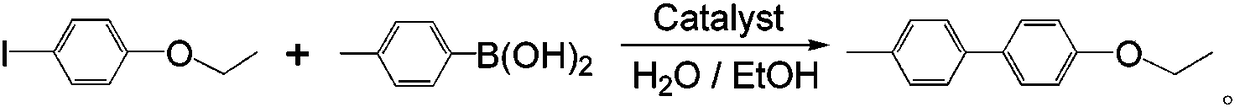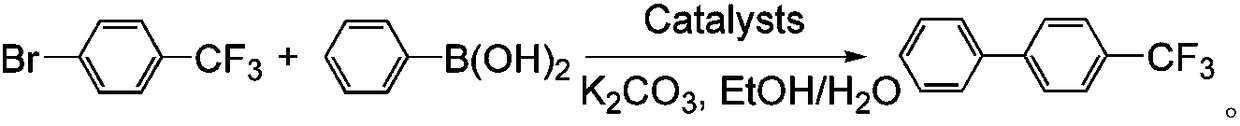A kind of sulfaquinoxaline cobalt-nickel nanocomposite material and preparation method thereof
A nano-composite material, quinoxaline cobalt-nickel technology, which is applied in the field of metal-organic nano-composite material catalysis, can solve the problems of cumbersome preparation steps, coordination of nickel catalysts, and reduced catalytic activity, and achieves broad application prospects, stable performance, Highly reactive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
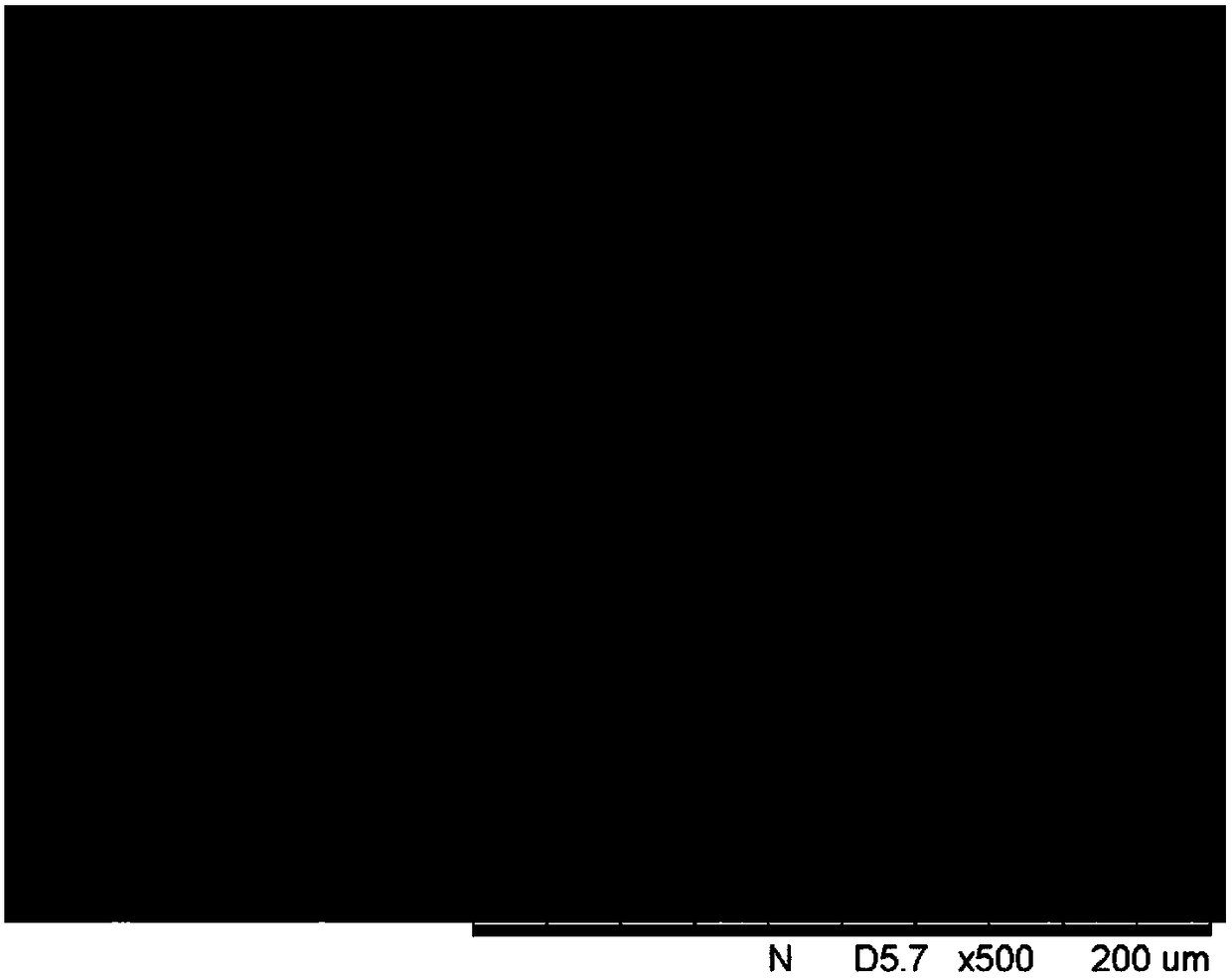
[0022] Weigh 0.1mmol of CoCl 2 ·6H 2 O and 0.9 mmol NiCl 2 ·6H 2 O was dissolved in 10mL water and stirred rapidly for 15min; 2.0mmol sulfaquinoxaline was weighed and dissolved in 10mL ethanol, stirred rapidly for 15min; under stirring, cobalt salt and nickel salt aqueous solution were mixed to form a mixed solution, and the mixed solution Add it dropwise to the ethanol solution of the sulfaquinoxaline ligand, stir at room temperature for 15 minutes, then microwave for 15 minutes, and ultrasonically disperse for 15 minutes to obtain a precipitated product. The precipitated product was centrifuged, washed with deionized water and ethanol three times, and dried to obtain the sulfaquinoxaline cobalt-nickel nanocomposite material. The morphology of the nanocomposite was observed with a scanning electron microscope, as figure 1 shown.
Embodiment 2
[0024] Weigh 0.9mmol of Co(NO 3 ) 2 ·6H 2 O and 0.1mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 10mL water and stirred rapidly for 15min; 4.0mmol sulfaquinoxaline was weighed and dissolved in 20mL ethanol, stirred rapidly for 15min; under stirring, cobalt salt and nickel salt aqueous solution were mixed to form a mixed solution, and the mixed solution Add it dropwise to the ethanol solution of the sulfaquinoxaline ligand, stir at room temperature for 30 minutes, then microwave for 15 minutes, and ultrasonically disperse for 15 minutes to obtain a precipitated product. The precipitated product was centrifuged, washed with deionized water and ethanol three times, and dried to obtain the sulfaquinoxaline cobalt-nickel nanocomposite material.
Embodiment 3
[0026] Weigh 0.3mmol of CoSO 4 ·6H 2 O and 0.7 mmol NiSO 4 ·6H 2 O was dissolved in 10mL water and stirred rapidly for 15min; 2.0mmol sulfaquinoxaline was weighed and dissolved in 20mL ethanol, stirred rapidly for 15min; under stirring, cobalt salt and nickel salt aqueous solution were mixed to form a mixed solution, and the mixed solution Add dropwise to the ethanol solution of sulfaquinoxaline ligand, stir at room temperature for 30 minutes, react in microwave for 30 minutes, and disperse ultrasonically for 15 minutes to obtain a precipitated product. The precipitated product was centrifuged, washed with deionized water and ethanol three times, and dried to obtain the sulfaquinoxaline cobalt-nickel nanocomposite material.
[0027] With embodiment 1, the sulfaquinoxaline cobalt-nickel nanocomposite material prepared by embodiment 2 and embodiment 3 is example, carry out catalytic reaction:
[0028] Use the sulfaquinoxaline cobalt-nickel nanocomposite prepared in the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com