Method for separating alogliptin benzoate and its enantiomers by high performance liquid chromatography
A high-performance liquid chromatography, enantiomer technology, applied in the field of medicine, can solve problems such as low activity, and achieve the effect of ensuring effectiveness and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
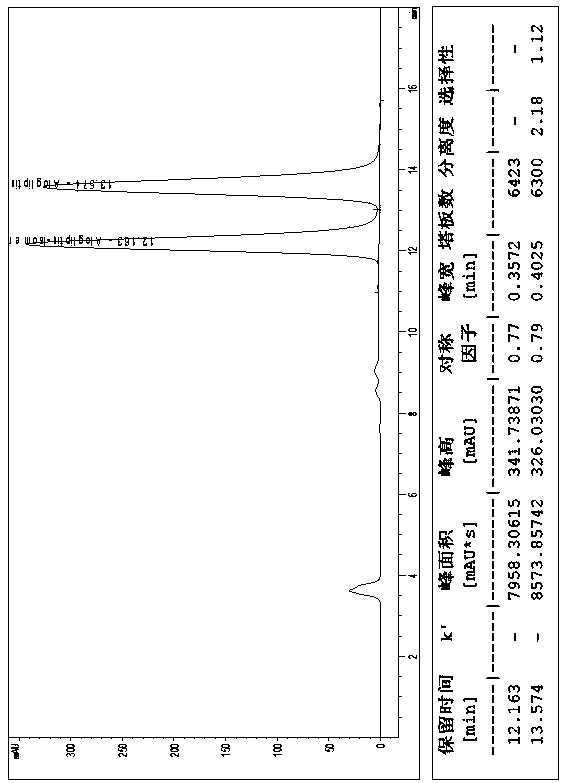
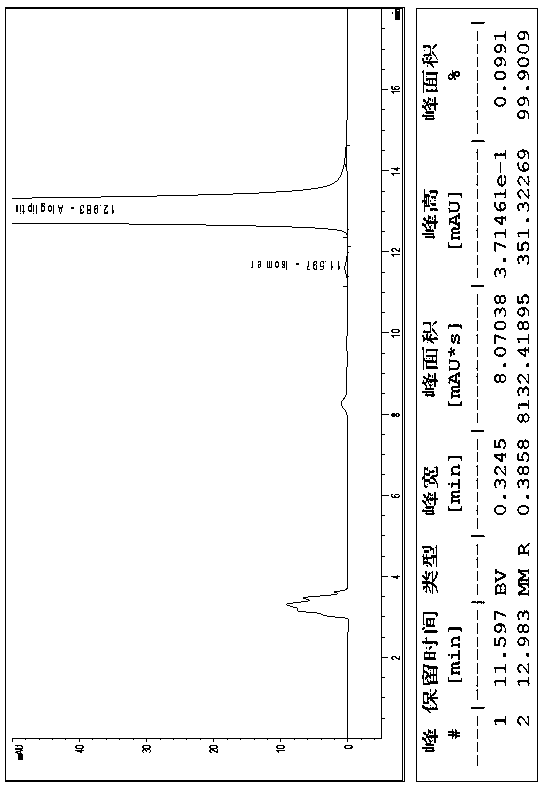
[0018] Embodiment 1, a kind of method of high-performance liquid chromatography resolution alogliptin benzoate and its enantiomer: the alogliptin benzoate containing alogliptin benzoate and its enantiomer The raw material drug is dissolved in the diluent to a concentration of 0.1 mg / mL; cellulose-tris(3,5-dichlorophenylcarbamate) covalently bonded to the surface of silica gel is used as the stationary phase, and n-hexane, ethyl acetate , dehydrated ethanol and triethylamine mixed solution are mobile phases, and alogliptin benzoate and its enantiomers are resolved with a high performance liquid chromatography system; the diluent is selected from one of acetonitrile, methanol and ethanol one or a mixture of several. The cellulose-tris(3,5-dichlorophenylcarbamate) chiral column is a bonded chiral chromatography column CHIRALPAK IC. The flow rate of the mobile phase is 0.5mL / min, the temperature of the chromatographic column is 25°C, and the detection wavelength is 220nm. The in...
Embodiment 2
[0019] Embodiment 2, a kind of method of high-performance liquid chromatography resolution alogliptin benzoate and its enantiomer: alogliptin benzoate containing alogliptin benzoate and its enantiomer The raw material drug was dissolved in the diluent to a concentration of 2 mg / mL; cellulose-tris(3,5-dichlorophenylcarbamate) covalently bonded to the surface of silica gel was used as the stationary phase, and n-hexane, ethyl acetate, The mixed solution of absolute ethanol and triethylamine is the mobile phase, and alogliptin benzoate and its enantiomers are resolved with a high-performance liquid chromatography system; the diluent is selected from one of acetonitrile, methanol, and ethanol. Or a mixture of several components. The cellulose-tris(3,5-dichlorophenylcarbamate) chiral column is a bonded chiral chromatography column CHIRALPAK IC. The flow rate of the mobile phase is 2.0 mL / min, the temperature of the chromatographic column is 45° C., and the detection wavelength is ...
Embodiment 3
[0020] Embodiment 3, a kind of method of high-performance liquid chromatography resolution alogliptin benzoate and its enantiomer: the alogliptin benzoate containing alogliptin benzoate and its enantiomer The raw material drug was dissolved in the diluent to a concentration of 1 mg / mL; the stationary phase was cellulose-tris(3,5-dichlorophenylcarbamate) covalently bonded to the surface of silica gel, and n-hexane, ethyl acetate, The mixed solution of absolute ethanol and triethylamine is the mobile phase, and alogliptin benzoate and its enantiomers are resolved with a high-performance liquid chromatography system; the diluent is selected from one of acetonitrile, methanol, and ethanol. Or a mixture of several components. The flow rate of the mobile phase described in 1 is 1.0mL / min, the temperature of the chromatographic column is 35°C, and the detection wavelength is 270nm. The sample injection volume is 10L. In the mobile phase, calculated by volume ratio, n-hexane: ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com