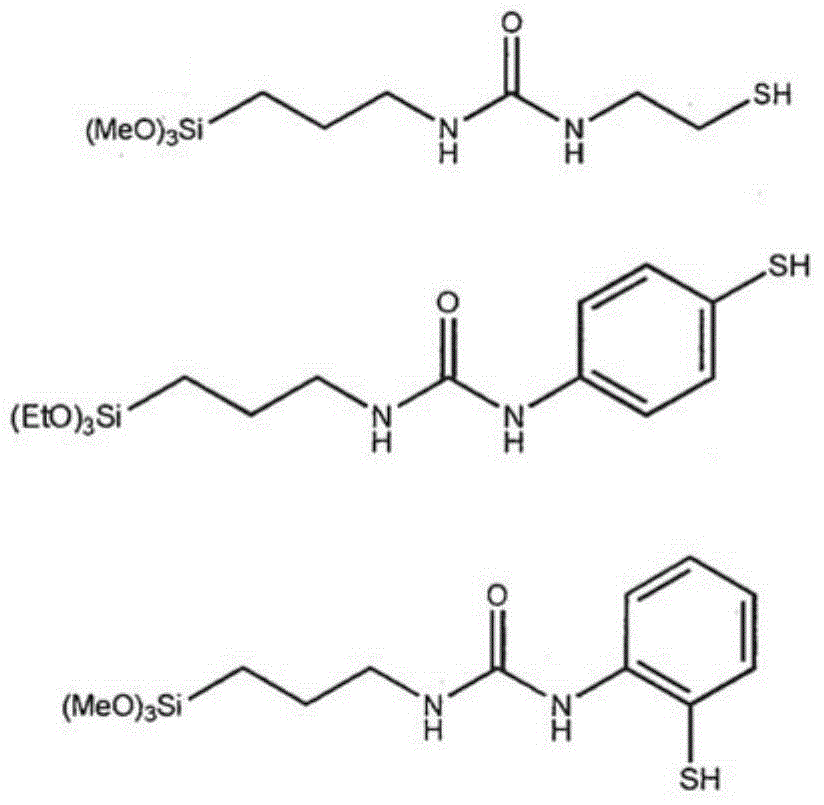
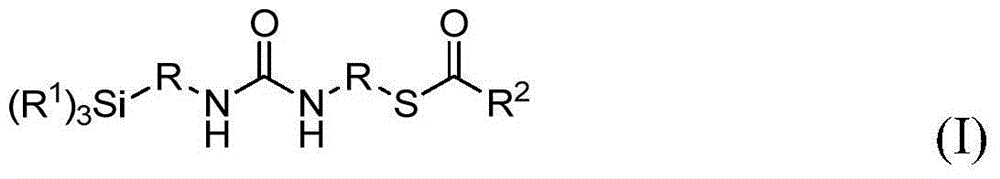
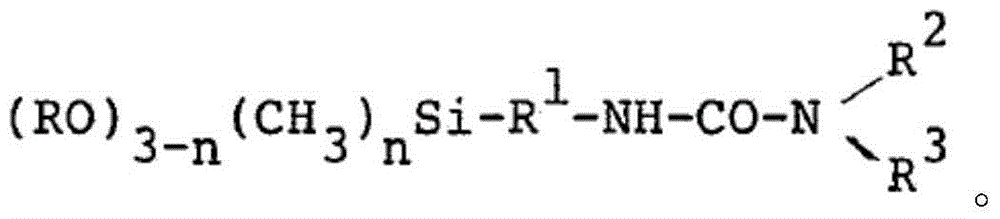Urea-containing mercaptosilanes, process for preparation thereof and use thereof
A mercaptosilane, hydrocarbon-based technology, applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as poor processing characteristics, low network density, and poor wet-slip properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Embodiment 1: by (EtO) 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -Cl and KSAc preparation (EtO) 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -S-CO-CH 3
[0166] At the beginning will (EtO) 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -Cl (81.52 g, 0.25 mol, 1.00 equiv) was charged into ethanol (85 ml) in a 500 ml three-necked flask with a stirrer, reflux condenser and internal thermometer. Potassium thioacetate (28.48 g, 0.25 mol, 1.00 equiv) was added and the mixture was heated to reflux. After 3.5 hours reaction time, the mixture was cooled to room temperature and the suspension was filtered. The filtrate was freed of solvent on a rotary evaporator and dried under reduced pressure. (EtO) was obtained as a light brown solid 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -S-CH 3Product (81.64 g, 89% of theory).
[0167] 1 H NMR (δ ppm ,500MHz,CDCl 3 ):0.64(2H,t),1.24(9H,t),1.61(2H,m),2.35(3H,s),3.01(2H,t),3.16(2H,t),3.34(2H,t) ,3.82(6H,q),4.5-7.0(2H,br);
...
Embodiment 2
[0169] Embodiment 2: by (EtO) 3 Si-CH 2 CH 2 CH 2 -NH 2 、OCN-CH 2 CH 2 -Cl and KSAc preparation (EtO) 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -S-CO-CH 3
[0170] 3-Aminopropyltriethoxysilane (132.82 g, 0.60 mol, 1.00 equiv) was initially charged to ethanol (300 ml) in a three-necked flask equipped with a precision glass stirrer, internal thermometer, dropwise Funnel and reflux condenser, and cooled to -78°C. 2-Chloroethyl isocyanate (63.34 g, 0.60 mol, 1.00 equiv) was added dropwise at -78°C to -68°C over 1.75 hours, then the mixture was heated to 50°C. Potassium thioacetate (68.53 g, 0.60 mol, 1.00 equiv) was added in five portions, and the mixture was heated to reflux. After 2.25 hours reaction time, the mixture was cooled to room temperature and the suspension was filtered. The filtrate was freed of solvent on a rotary evaporator and dried under reduced pressure. (EtO) was obtained as a waxy white solid 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -S-CH 3...
Embodiment 3
[0173] Embodiment 3: by (EtO) 3 Si-CH 2 CH 2 CH 2 -NCO, HCl·H 2 N-CH 2 CH 2 -Cl and KSAc preparation (EtO) 3 Si-CH 2 CH 2 CH 2 -NH-CO-NH-CH 2 CH 2 -S-CH 3
[0174] 2-Chloroethylamine hydrochloride (73.86 g, 0.70 mol, 1.00 eq) was initially charged to ethanol (2.0 l) in a 4 l three-necked flask with precision glass stirrer, internal thermometer, drop Add a funnel and reflux condenser, and cool to -78°C, then add sodium ethoxide (226.83 g, 0.70 mol, 1.00 equiv, 21% in ethanol). 3-Isocyanatopropyl(triethoxysilane) (173.15 g, 0.70 mol, 1.00 equiv) was then added dropwise at -78°C to -65°C over 3 hours, and the mixture was then heated to 50°C . Potassium thioacetate (79.95 g, 0.70 mol, 1.00 equiv) was added in five portions and the mixture was heated to reflux. After 4 hours reaction time, the mixture was cooled to room temperature and the suspension was filtered. The filtrate was freed of solvent on a rotary evaporator and dried under reduced pressure. Obtained a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com