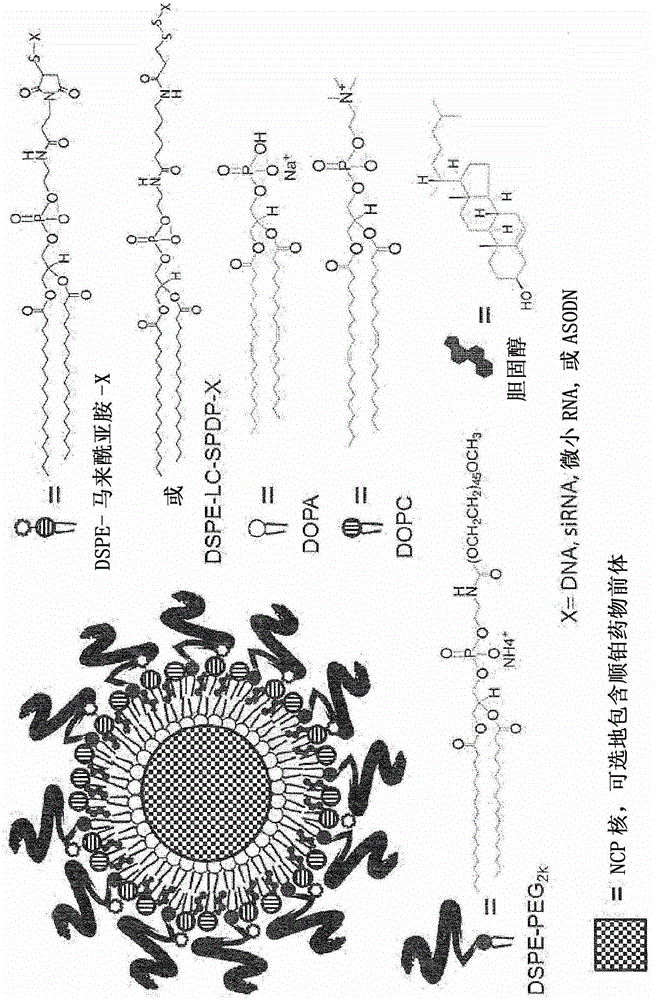
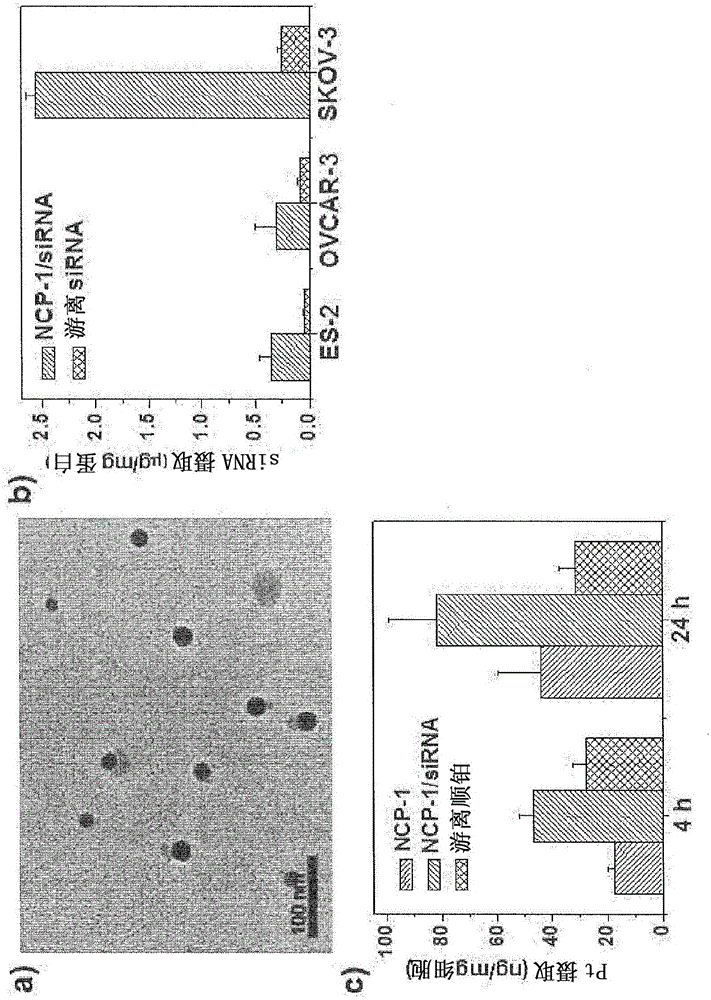
Nanoscale carriers for the delivery or co-delivery of chemotherapeutics, nucleic acids and photosensitizers
A nanoscale, chemotherapeutic technology, applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve problems such as limiting PDT efficacy, nucleic acid degradation, and short-lived nucleic acid effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0243] Nanoscale coordination polymers with cisplatin or oxaliplatin and with electrostatic interaction on the particle surface RNA adsorbed on
[0244] 1.1. Synthesis of cisplatin prodrug (cisPtBp):
[0245]
[0246] Scheme 1. Structure of the bisphosphonate cisplatin prodrug.
[0247] The preparation of the bisphosphonate cisplatin prodrug, cisPtBp (Scheme 1) is described in International Application No. WO 2013 / 009701. More specifically, to cis,cis,trans-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ] (0.5 g, 1.5 mmol) in 2 mL of dimethylformamide (DMF) was added containing 4 equivalents of diethoxyphosphinyl (diethoxyphosphinyl) isocyanate (0.92 mL, 6.0 mmol) in 1 mL of DMF solution. The resulting mixture was stirred at room temperature in the dark for 12 h. The solution was filtered and the resulting bisphosphonate complex was precipitated by adding diethyl ether and washed at least twice with diethyl ether to remove residual DMF. Yield: 80%. DMSO-d 6 middle 1 H NMR: δ8.61 (...
Embodiment 2
[0312] Chemotherapeutic agents loaded sequentially in porous nanoparticle coordination polymers
[0313]
[0314] Scheme 3. Cisplatin prodrug cis,cis,trans-[Pt(NH 3 ) 2 Cl 2 (OEt)(OCOCH 2 CH 2 COOH)] synthesis.
[0315] The cisplatin prodrug cis,cis,trans-[Pt(NH 3 ) 2 Cl 2 (OEt)(OCOCH 2 CH 2 COOH)]. More specifically, cisplatin was reacted with hydrogen peroxide in ethanol to provide an intermediate product with a hydroxyl group and one ethoxy ligand. The intermediate is then reacted with succinic anhydride to provide the prodrug.
[0316]
[0317] Scheme 4. Synthesis of amino-triphenyldicarboxylic acid (amino-TPDC) ligands.
[0318] Due to the high connectivity of SBU and the strong interaction between zirconium and oxygen, based on Zr 6(μ 3 -O) 4 (μ 3 -OH) 4 NCPs of secondary building units (SBU) and dicarboxylic acid bridging ligands are highly porous and stable in aqueous environments. This material is called UiO, after the original family discove...
Embodiment 3
[0325] Materials and methods for nanoparticle coordination polymers with photosensitizers and chemotherapeutic agents
[0326] 3.1. Materials, cell lines and animals:
[0327] Unless otherwise stated, all starting materials were purchased from Sigma-Aldrich Company (St.Louis, Missouri, United States of America) and Fisher (Thermo Fisher Scientific, Waltham, Massachusetts, United States of America), and used without further purification. 1,2-Dioleoyl-sn-glycero-3-phosphate sodium salt (DOPA), 1,2-distearoyl-sn-glycero-3-phosphorylcholine (DSPC), cholesterol and 1,2- Distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol) 2000] (DSPE-PEG2k) were both purchased from Avanti Polar Lipids (labaster, Alabama, United States of America).
[0328] Human head and neck cancer cell lines HNSCC135 (cisplatin-sensitive), SCC61 (cisplatin-sensitive), JSQ3 (cisplatin-resistant) and SQ20B (cisplatin-resistant) were developed by Dr. Stephen J. Kron (Department of Molecular ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com