High-molecular polymer containing spiropyran group and preparation method thereof and application thereof
A high-molecular polymer and spiropyran group technology, applied in the field of spiropyran-containing high-molecular polymer and its preparation, can solve the problems of performance and application range limitations, and achieve optimistic market application prospects, Simple method and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the presence of nitrogen, the spiropyran compound and the polymer were kept away from light and stirred at a stirring speed of 80r / min for 28 hours. After the reaction was completed, the mixture was filtered, and the filter cake was fully washed with tetrahydrofuran, ethanol and water successively, and vacuum dry. The vacuum degree of the vacuum drying is 0.06MPa, and the temperature is 45°C.
[0030] The spiropyran compound is a nitrogen-substituted C 3 Spiropyran derivatives of carboxylic acids.
[0031] The polymer is chitosan.
[0032] The molar ratio of the spiropyran compound and chitosan is 6:1.
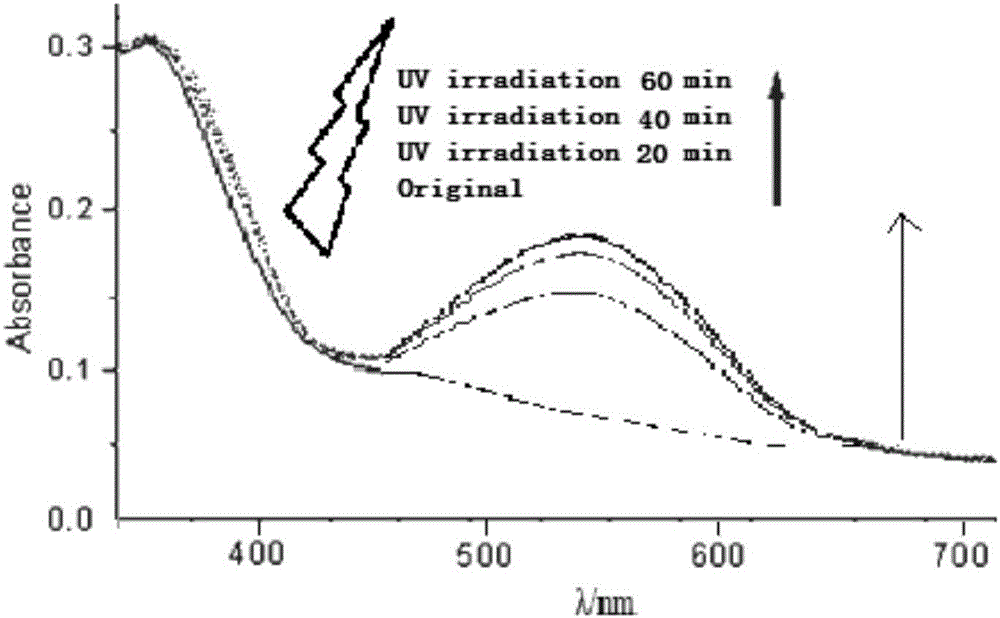
[0033] After preparing the spiropyran-modified chitosan, 1 mg of the product was dissolved in 10 mL of ethanol, filtered through a 0.22 mm filter membrane, and the photostimuli response performance was tested by ultraviolet-visible spectroscopy (UV-Vis). determined, as figure 2 As shown in the results, the modified compound has no absorption at around 587nm bef...
Embodiment 2
[0036]In the presence of nitrogen, the spiropyran compound, polymer, and solvent were stirred and reacted at a stirring speed of 80r / min for 48 hours in the presence of nitrogen. After the reaction was completed, the mixture was filtered, and the filter cake was thoroughly washed with tetrahydrofuran, ethanol, and water. Wash and dry in vacuo. The vacuum degree of the vacuum drying is 0.07MPa, and the temperature is 55°C.
[0037] The spiropyran compound is a nitrogen-substituted C 4 Hydroxy spiropyran derivatives.
[0038] The polymer is hyaluronic acid.
[0039] The solvent is N,N-dimethylformamide (DMF), and the dosage is 4:1.
[0040] The molar ratio of the spiropyran compound and hyaluronic acid is 6:1.
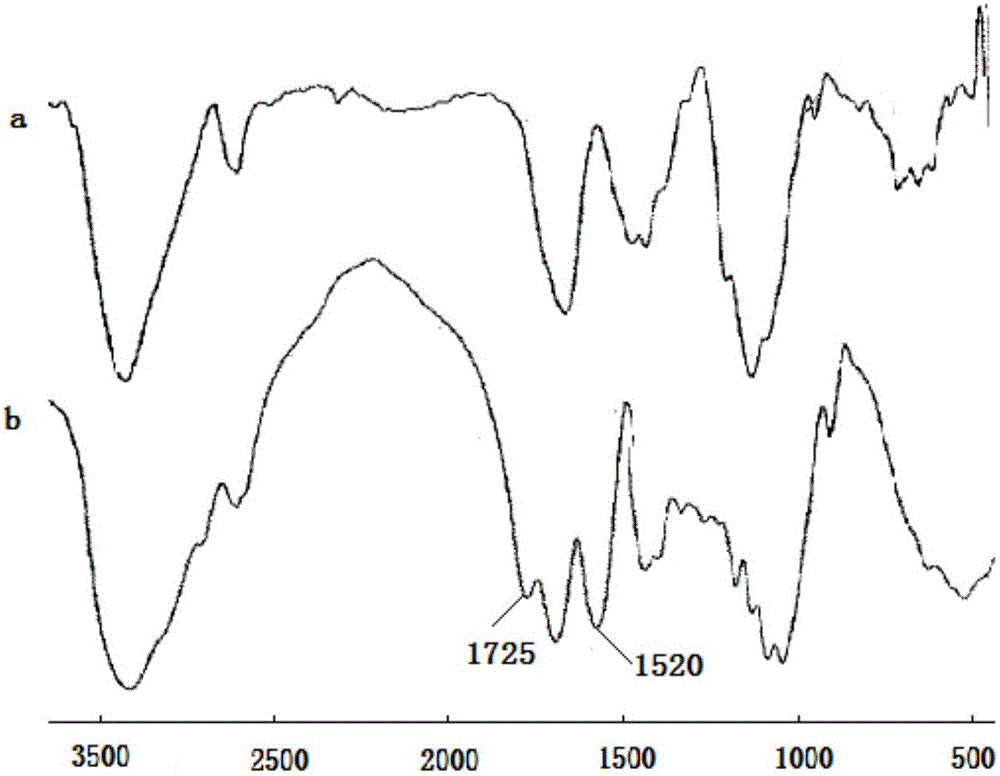
[0041] After preparing the spiropyran-modified hyaluronic acid, use the KBr tablet method, and use the Fourier transform infrared spectrometer to scan the infrared spectrum of the compound before and after modification. The wavelength range is from 400 to 4000 cm -1...
Embodiment 3
[0044] The spiropyran compound, polymer, solvent, and condensing agent were stirred and reacted for 36 hours at a stirring speed of 60r / min in the presence of nitrogen, protected from light, and after the reaction was completed, the mixture was filtered, and the filter cake was washed with tetrahydrofuran, ethanol , fully washed with water, and dried in vacuum.
[0045] The spiropyran compound is a carboxyl-containing spiropyran derivative, and the carboxyl group is on the spiropyran ring. The polymer is cellulose. The solvent is tetrahydrofuran in an amount of 5:1. The condensing agent is 1-ethyl-3(3-dimethylpropylamine) carbodiimide (EDCI), and the dosage is 0.25:1. The molar ratio of the spiropyran compound to the cellulose is 8:1. The vacuum degree of the vacuum drying is 0.08MPa, and the temperature is 50°C.
[0046] After preparing the spiropyran-modified cellulose, use the KBr tablet method, and use the Fourier transform infrared spectrometer to scan the infrared sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com