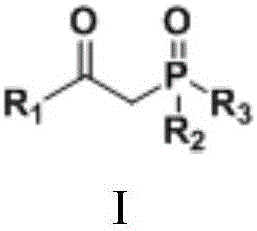
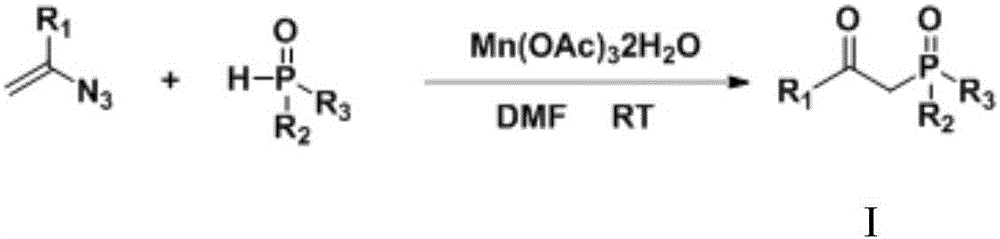
Preparation method of beta-ketone substituted phosphate ester compound
A technology for phosphate esters and compounds, which is applied in the field of preparation of phosphate ester compounds, can solve the problems of harsh reaction conditions, limited range of substrate substituents, etc., and achieves the effects of simple operation, cheap reaction raw materials, and reasonable design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
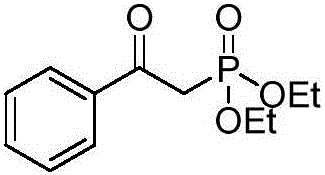
[0032] Embodiment 1: the preparation of (2-oxo-2-phenylethyl) diethyl phosphite
[0033] Add (1-azidovinyl)benzene (1mmol, 145mg) and diethyl phosphite (1.0mmol, 138mg) into a sealed glass tube, then add 2.0mL of DMF, after the addition, react at room temperature, TLC The reaction was detected (ethyl acetate:petroleum ether=1:5), and the reaction ended after 6 hours. The reaction solution was concentrated in vacuo, and the concentrated solution was extracted with ethyl acetate (20mL×3). The combined organic phases were washed twice with water and once with saturated brine, dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a residue, which was subjected to column chromatography. Analysis and separation (eluent: ethyl acetate:petroleum ether=1:4) gave a yellow liquid with a yield of 92%.
[0034] Its structural formula is:
[0035]
[0036] Yellow oil, 236mg, yield 92%. 1 H NMR (500MHz, CDCl 3 )δ8.01(d,J=7.0Hz,2H),7.80-7.57(m,1H),7.48(t,J=8.0Hz,2H),4...
Embodiment 2
[0037] Embodiment 2: Preparation of (2-(4-methylphenyl)-2-oxoethyl) diethyl phosphite
[0038] The synthesis method is the same as in Example 1, except that the raw material 1-azidenylbenzene is replaced with 1-azidoenyl-4-methylbenzene. Pale yellow liquid, yield 88%, its structural formula is:
[0039]
[0040] Yellow oil, yield 88%. 1 H NMR (500MHz, CDCl 3 )δ7.91(d, J=8.0Hz, 2H), 7.28-7.27(m, 2H), 4.17-4.11(m, 4H), 3.61(d, J=22.5Hz, 2H), 2.42(s, 3H ),1.28(t,J=7.0Hz,6H). 13 C NMR (125MHz, CDCl 3 )δ191.6(d, J=7.5Hz), 144.8, 134.2, 129.4, 129.3, 62.8(d, J=6.25Hz), 38.5(d, J=130Hz), 21.81, 16.4(d, J=6.25Hz ).HRMS(ESI):m / z calcd for(C 13 h 19 o 4 P+H) + :271.1094; found: 271.1090.
Embodiment 3
[0041] Embodiment 3: Preparation of (2-(3-bromo-4-ethoxyphenyl) 2-oxoethyl) diethyl phosphite
[0042] The synthesis method is the same as in Example 1, except that the raw material 1-azidenylbenzene is replaced with 1-azidonyl-(3-bromo-4-ethoxyl)benzene. Yellow liquid, yield 79%, its structural formula is:
[0043]
[0044] Yellow oil, yield 79%. 1 H NMR (500MHz, CDCl 3 )δ8.21(d, J=2.0Hz, 1H), 7.96(dd, J=8.5, 2.0Hz, 2H), 6.91(d, J=9.0Hz, 2H), 4.20-4.11(m, 4H), 3.55(d, J=22.5Hz, 2H), 1.50(t, J=7.0Hz, 3H), 1.29(t, J=7.0Hz, 6H). 13 C NMR (125MHz, CDCl 3 )δ189.4(d, J=6.25Hz), 159.8, 134.7, 130.6, 130.5, 112.4, 112.0, 65.3, 62.8(d, J=6.25Hz), 38.6(d, J=129Hz), 16.4(d, J=6.25Hz), 14.62. HRMS (ESI): m / z calcd for (C 14 h 20 BrO 5 P+H) + :379.0304; found: 379.0306.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com