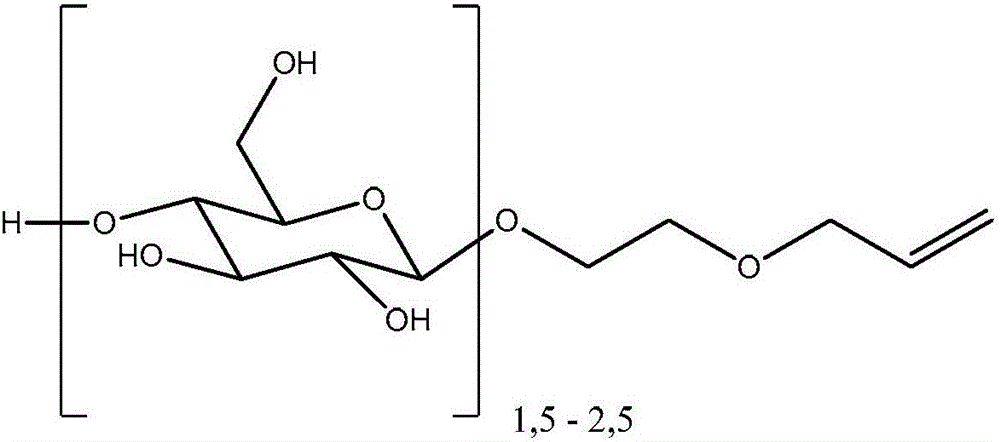
Polyorganosiloxane gels having glycoside groups
A polysiloxane gel, polysiloxane technology, which is applied to skin care preparations, cosmetic preparations, pharmaceutical formulations, etc., can solve the problems of high cost and unsatisfactory cosmetic application, and achieve good sensory performance, superior The effect of sliding and good skin feel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-14 and comparative example C1-C6
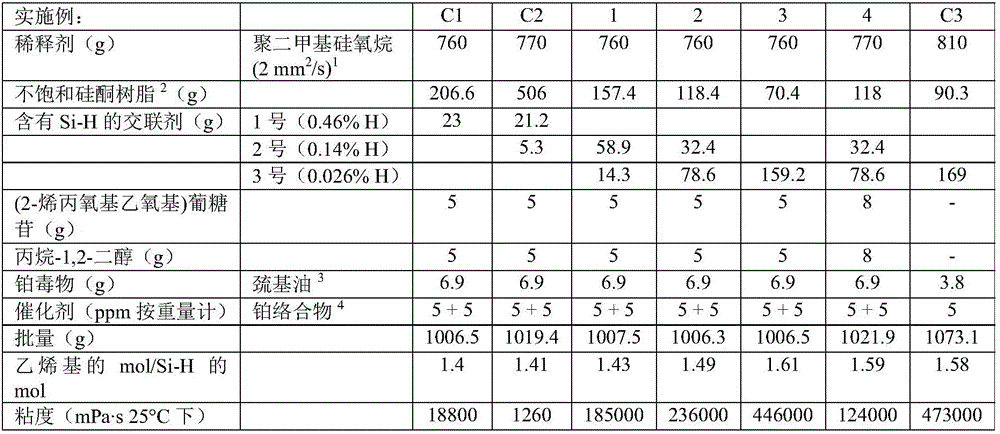
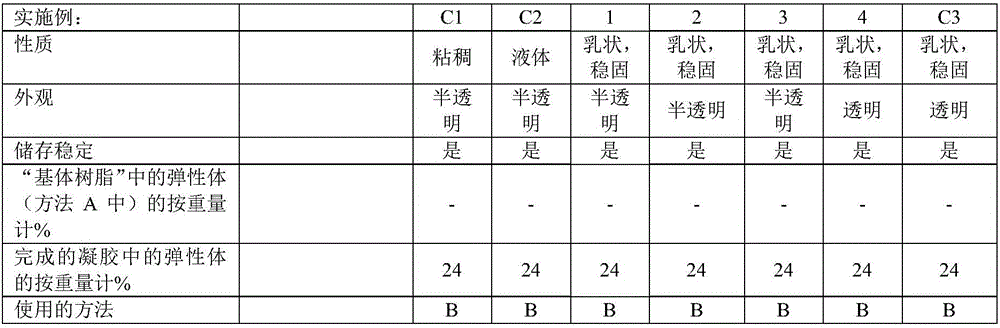
[0180] According to methods A and B, a series of gels were produced. The properties of the Si—H functionalized crosslinkers used in the examples and comparative examples are shown in Table 1. Crosslinkers 1 and 2 (Table 1) were added in Step H1; Crosslinker 3 (Table 1) was added in Step H2. The substances used, their amounts and the properties of the gels produced are shown in Tables 2 to 5 below.
[0181] Table 1 - Properties of Si-H-containing crosslinkers used in Examples 1-14 and Comparative Examples C1-C6:
[0182] Numbering
Distribution a:(b+c)
sum of a+b
Viscosity (mm 2 / s at 25℃)
%H
1
2:1
138
331
0.47
2
9:1
60
58
0.14
3
55:1
134
321
0.026
[0183] Examples 1-7 are examples of inventive gels in which a crosslinker with very low Si-H group content is used as the sole crosslinker or in combination with additional crosslinkers. The d...
Embodiment 15-21 and comparative example C7-C8
[0220] Examples 15-21 and Comparative Examples C7-C8: Mixtures containing water
[0221] Aqueous mixtures were produced using the inventive organopolysiloxane gels according to Examples 1-4 and 10, and the gels from Comparative Examples C3 and C6. For this purpose, variable amounts of water are added to the gel. use water The mixer was introduced partially by shear at 1000 rpm and room temperature. Components and results are listed in Tables 6 and 7.
[0222] Examples 15, 16, 18, 20 and 21 show single-phase, homogeneous, stable, milky, translucent mixtures of organopolysiloxane gels of the invention and water. Examples 17 and 19 show saturated mixtures. They are stable, translucent and milky, but individual fine droplets are present on the surface. This is an indication that the network of the organopolysiloxane gel of the invention is saturated with solvent. Now the gel is no longer able to absorb any additional water. Comparative examples C3 and C6 are milky organopo...
Embodiment 22-27 and comparative example C9 and C10
[0230] Examples 22-27 and comparative examples C9 and C10: mixtures containing glycerol
[0231] Glycerol-containing mixtures were produced using the inventive organopolysiloxane gels according to Examples 1-4, 10 and 11, as well as the gels from Comparative Examples C3 and C6. For this purpose, variable amounts of glycerol were added to the gel. Use glycerol The mixer was introduced partially by shear at 1000 rpm and room temperature. Components and results are listed in Table 8.
[0232]Examples 22-27 show single-phase, homogeneous, stable, milky, white mixtures of organopolysiloxane gels of the present invention with glycerol. Comparative examples C3 and C6 are milky organopolysiloxane gels produced without additional use of the compound (1b) according to the invention, ie without glycosidic residues and hydrosilylatable end groups. First they absorb very small amounts, ie less than 5% by weight, of glycerol, and then they separate into two phases (comparative examples...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| iodine value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com