Polysubstituted thienoindole derivative and preparation method thereof
A technology of indole derivatives and multiple substitutions, applied in organic chemistry and other fields, can solve the problems of large environmental pollution, expensive catalytic metals, and high toxicity of reagents, and achieve the effects of scientific and reasonable synthesis methods, simple synthesis methods, and easy-to-use products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
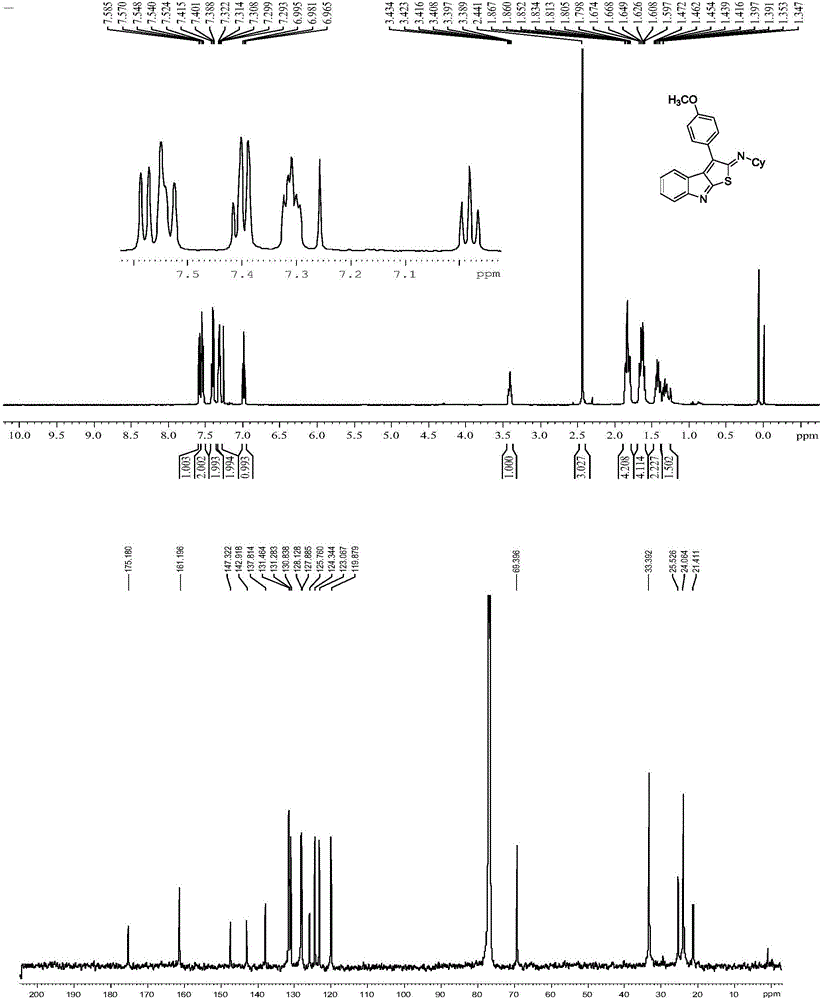
[0024] Example 1: (Z)-N-cyclohexyl-3-phenyl-2H-thieno[2,3-b]indol-2-amine (R in structural formula I 1 = cyclohexyl, R 2 = phenyl, R 3 = Hydrogen atom, R 4 = Hydrogen atom)
[0025] Add 2-phenylethynyl-phenylisothiocyanate (1.0mmol, 235mg), cyclohexylisonitrile (1.2mmol, 153uL) and nickel acetylacetonate (0.003mmol, 0.88mg) to a 25mL round bottom flask, add 3.0 mL of redistilled tetrahydrofuran, sealed and placed in an oil bath at 80°C for 5 hours. After the reaction was complete, cool to room temperature, extract the system with ethyl acetate, then evaporate the solvent with a rotary evaporator, and separate the residue by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=20 / 1) The red solid product (Z)-N-cyclohexyl-3-phenyl-2H-thieno[2,3-b]indol-2-amine 336 mg with a purity greater than 99% was obtained. The isolated yield was 96%.
[0026] Structural identification of (Z)-N-cyclohexyl-3-phenyl-2H-thieno[2,3-b]indol-2-amine:
[0027] 1 HNM...
Embodiment 2
[0028] Example 2: (Z)-N-cyclohexyl-3-(4-fluorophenyl)-2H-thieno[2,3-b]indole-2-imine (R in structural formula I 1 = cyclohexyl, R 2 = p-fluorophenyl, R 3 = Hydrogen atom, R 4 = Hydrogen atom)
[0029] To a 25 mL round bottom flask was added (3-fluorophenyl)ethynyl-phenylisothiocyanate (1.0 mmol, 253 mg), cyclohexylisonitrile (1.2 mmol, 153 uL) and nickel acetylacetonate (0.003 mmol, 0.88 mg ), add 3.0 mL redistilled tetrahydrofuran, seal the mouth and put it in an oil bath at 80°C for 5 hours. After the reaction was complete, cool to room temperature, extract the system with ethyl acetate, then evaporate the solvent with a rotary evaporator, and separate the residue by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=20 / 1) The product (Z)-N-cyclohexyl-3-(4-fluorophenyl)-2H-thieno[2,3-b]indole-2-imine was obtained as a red solid with a purity greater than 99% 294 mg isolated yield 95%.
[0030] Structural identification of (Z)-N-cyclohexyl-3-...
Embodiment 3
[0032] Example 3: (Z)-N-cyclohexyl-5-fluoro-3-phenyl-2H-thieno[2,3-b]indol-2-amine (R in structural formula I 1 = cyclohexyl, R 2 = phenyl, R 3 = fluorine atom, R4 = Hydrogen atom)
[0033] Into a 25 mL round bottom flask was added 4-fluoro-2-(phenylethynyl)-phenylisothiocyanate (1.0 mmol, 253 mg), cyclohexylisonitrile (1.2 mmol, 153 uL) and nickel acetylacetonate (0.003 mmol ,0.88mg), add 3.0mL redistilled tetrahydrofuran, seal the mouth and put it in an oil bath at 80°C for 5h. After the reaction was complete, cool to room temperature, extract the system with ethyl acetate, then evaporate the solvent with a rotary evaporator, and separate the residue by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=20 / 1) The product (Z)-N-cyclohexyl-5-fluoro-3-phenyl-2H-thieno[2,3-b]indol-2-amine 330mg with a purity of more than 99% was obtained. The isolated yield was 93% .
[0034] Structural identification of (Z)-N-cyclohexyl-5-fluoro-3-phenyl-2H-thie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com