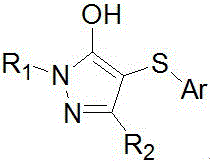
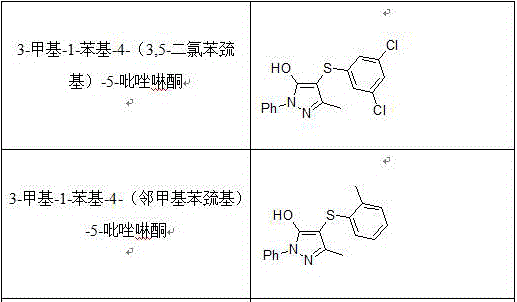
Preparation method of 4-thio-pyrazolone derivative
A technology of pyrazolone and derivatives, applied in the field of organic synthesis, can solve the problems of low yield, poor substrate applicability, difficult operation, etc., and achieve the effect of easy-to-obtain and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Add 1,3-dimethylpyrazolone (0.5mmol), 4-methylbenzenesulfonyl chloride (0.6mmol), KI (0.1mmol), and triphenylphosphine (1.0mmol) in sequence in a 15mL pressure-resistant tube and 1,4-dioxane (1 mL), then heated to 80 o C reaction, after spotting the plate to detect the completion of the reaction, gradient elution was carried out by dichloromethane:methanol volume ratio of 40:1–20:1. A white solid was obtained in 85% yield. NMR data: 1 HNMR (400MHz, d6 DMSO)δ11.29(s,1H),7.06(d,J=8.0Hz,2H),6.89(d,J=7.6Hz,2H),3.48(s,3H),2.22(s,3H),1.98 (s,3H)
[0065]
Embodiment 2
[0067] Add 1,3-dimethylpyrazolone (0.5mmol), 4-methoxybenzenesulfonyl chloride (0.6mmol), KI (0.1mmol), triphenylphosphine (1.0mmol ) and 1,4-dioxane (1 mL), then heated to 60 oC reaction, spot plate detection After the reaction is complete (the reaction time is 8-24 hours), the gradient elution is carried out by dichloromethane: methanol volume ratio of 40:1-20:1. A pale yellow solid was obtained with a yield of 83%. NMR data: 1 HNMR (400MHz, d6 DMSO) δ11.32(s,1H),6.98(s,2H),6.86(s,2H),3.70(s,3H),3.47(s,3H),2.00(s,3H).
[0068]
Embodiment 3
[0070] Add 1,3-dimethylpyrazolone (0.5mmol), 4-cyanobenzenesulfonyl chloride (0.6mmol), KI (0.1mmol), and triphenylphosphine (1.0mmol) in sequence in a 15mL pressure-resistant tube and 1,4-dioxane (1 mL), then heated to 100 o C reaction, spot plate detection After the reaction is complete (the reaction time is 8-24 hours), the gradient elution is carried out by dichloromethane: methanol volume ratio of 40:1-20:1. A white solid was obtained in 96% yield. NMR data: 1 HNMR (400MHz, d6 DMSO)δ11.58(s,1H),7.70(d,J=8.4Hz,2H),7.13(d,J=8.4Hz,2H),3.52(s,3H),1.98(s,3H)
[0071]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com