Fluorescent diagnosis and treatment reagent development for diagnosing and treating non-small cell lung cancer (NSCLC) and application of cells thereof
A technology of fluorescent probes, fluoropyrrole, which is applied in the field of fluorescent probes, can solve the problems of lack of tumor positioning groups, shortages, etc., and achieve the effect of improving the utilization rate of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of a compound of formula:
[0050] Under the protection of argon, D-biotin (1.215g, 5mmol) was dissolved in 90mL N,N-dimethylformamide, 4-dimethylaminopyridine (0.61g, 5mmol), 1-(3-bis Methylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.93g, 6mmol) and 1-hydroxybenzotriazole (0.067g, 0.5mmol) were added to the solution and reacted for 30 minutes, then the fluorophore ( 0.529g, 1mmol), after reacting at room temperature for 24 hours, wash with water and ethyl acetate (1:1 / v / v), obtain the ethyl acetate layer and spin dry the solvent with a rotary evaporator, and dissolve the obtained solid in dichloromethane , separated by silica gel column chromatography (200-300 mesh). The eluent was dichloromethane and methanol (50:1 / v / v), and the green fraction was collected.
[0051]Under the protection of argon, dissolve gefitinib (0.116g, 0.26mmol) in 100mL of dichloromethane and add bis(trichloromethyl)carbonate (1.539g, 5.2mmol) N,N-Diisopropylethylamine (1.00...
Embodiment 2
[0055] Compound of preparation formula two:
[0056] Under the protection of argon, dissolve (2) (0.194g, 0.31mmol) in 100mL of dichloromethane and add bis(trichloromethyl)carbonate (1.835g, 6.2mmol) Add N,N-diisopropylethylamine (1.200 g, 9.3 mmol) dropwise, and react at room temperature for 3 hours after the dropwise addition. After the reaction was completed, the solvent was evaporated to dryness on a rotary evaporator, and the obtained solid was dissolved in dichloromethane, and added dropwise to a solution containing N,N-diisopropylethylamine (0.040g, 0.31mmol) and fluorophore (0.132g, 0.25 mmol) in 50 mL of dichloromethane solution, reacted at room temperature for 36 hours. After the reaction, wash with water and dichloromethane (1:1 / v / v), spin dry the solvent with a rotary evaporator after obtaining the dichloromethane layer, dissolve the resulting solid in dichloromethane, and use aluminum oxide column chromatography (200-300 mesh) separation. The eluent was dichlor...
Embodiment 3
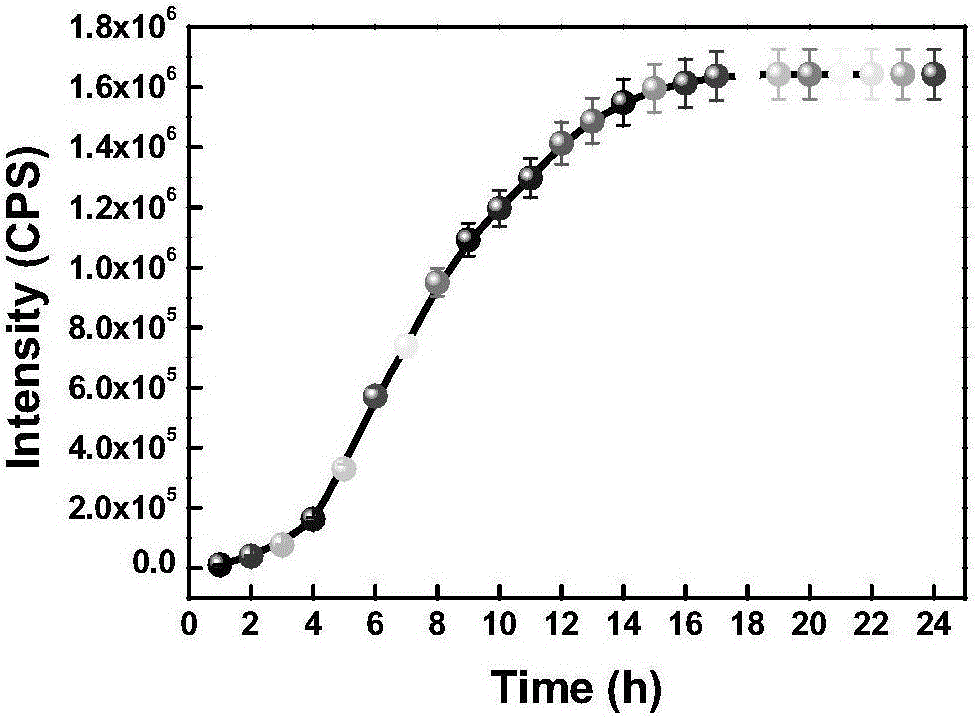
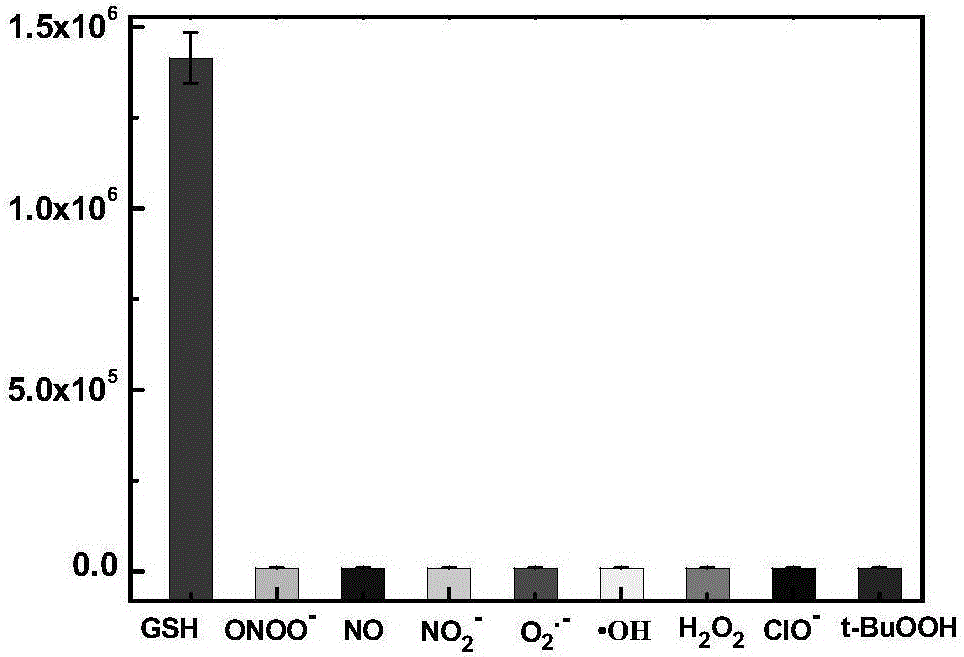
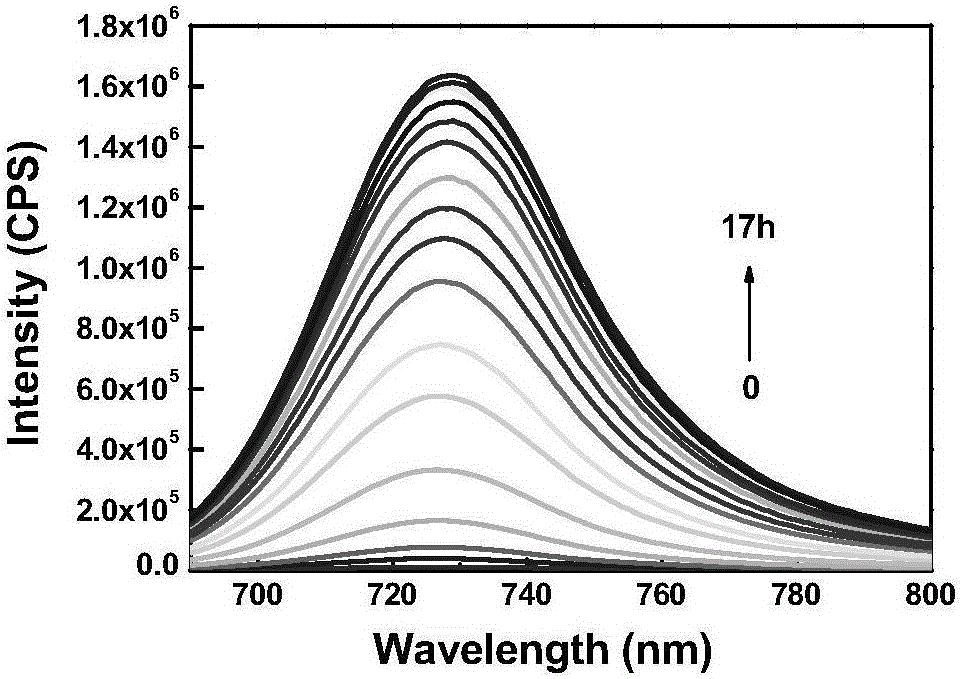
[0060] The prepared compound of formula one is used as a probe in the water system to simulate the physiological environment to detect the drug sustained release kinetics. Physiological conditions were simulated, and the following experiments were all carried out under the condition of pH=7.4 (HEPES buffer solution, the concentration was 40 mM). The compound of formula 2 is used at 10 μM, and the concentration of glutathione is 5 mM. like figure 1 As shown, the probe can achieve the purpose of drug sustained release.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com