Arctigenin ether derivatives and their preparation methods and uses
A technology of arctigenin ether and derivatives, which is applied in the field of drug synthesis, can solve the problems of low pass rate, high plasma protein binding rate, low bioavailability, blood-brain barrier, etc., and achieve the effect of easy synthesis and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
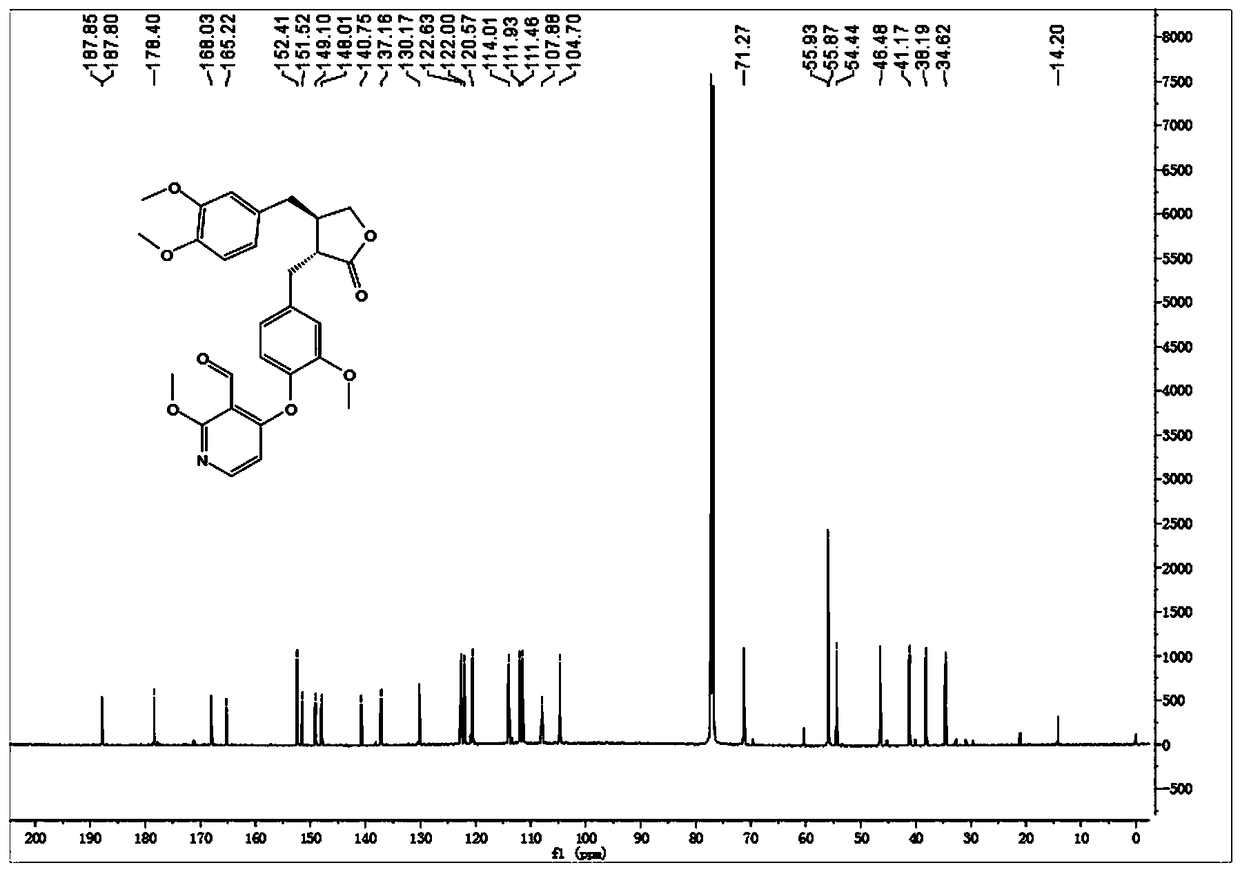
[0035] Embodiment 1: synthetic compound 1-1, please refer to figure 1 and figure 2
[0036] .
[0037] Dissolve arctigenin (50 mg, 0.134 mmol) in 2 ml of dry N,N-dimethylformamide (DMF), add potassium carbonate (37 mg, 0.268 mmol), stir at room temperature for 10 min, then add 4 -Iodo-2-methoxypyridine-3-carbaldehyde (42mg, 0.161 mmol), stirred at 120°C for 8h. After cooling to room temperature, 10 mL of ice water was added, and the solid precipitated out. Suction filtration, the filter cake was washed with 5% potassium bisulfate solution, dried in vacuo, and recrystallized from methanol to obtain 60 mg of white solid, with a yield of 88%. [α] D 25 =+3.9 (c1.0, CHCl 3 ); 1H-NMR (400 MHz, CDCl 3 ) δ 10.58 (s, 1H), 8.03 (d, J = 5.9 Hz,1H), 7.05 (d, J = 8.0 Hz, 1H), 6.85 – 6.70 (m, 3H), 6.62 – 6.52 (m, 2H), 6.13(d, J = 5.9 Hz, 1H), 4.20 (t, J = 8.2 Hz, 1H), 4.06 (s, 3H), 3.92 (t, J = 8.5Hz, 1H), 3.86 (s, 3H), 3.84 (s, 3H), 3.71 (s, 3H), 3.01 (d, J = 5.6 Hz...
Embodiment 2
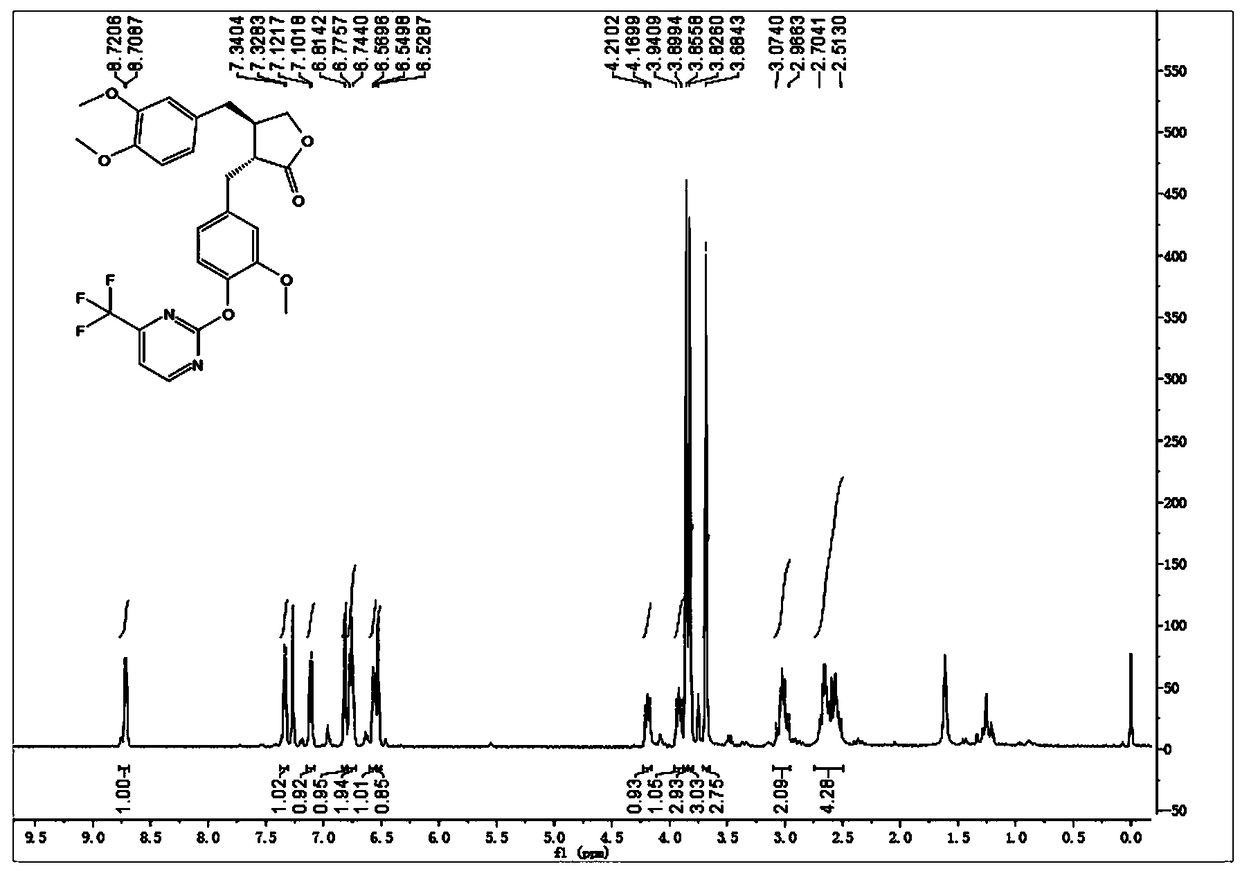
[0038] Embodiment 2: synthetic compound 1-2, please refer to image 3 and Figure 4
[0039] .
[0040] Dissolve arctigenin (50 mg, 0.134 mmol) in 2 ml of dry N,N-dimethylformamide (DMF), add potassium carbonate (37 mg, 0.268 mmol), stir at room temperature for 10 min, then add 2 -Bromo-3-trifluoromethylpyrimidine (37 mg, 0.161 mmol), stirred at 120°C for 8h. After cooling to room temperature, 10 mL of ice water was added, and the solid precipitated out. Suction filtration, the filter cake was washed with 5% potassium bisulfate solution, dried in vacuo, and methanol was recrystallized to obtain 64 mg of a white solid, with a yield of 92%; the white solid, [α ] D 20 = -1.5 (c 1.0, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 8.71 (d, J = 4.8 Hz, 1H), 7.33(d, J = 4.8 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H), 6.81 (s, 1H), 6.78 – 6.74 (m,2H), 6.59 – 6.54 (m, 1H), 6.53 (s, 1H), 4.23 – 4.15 (m, 1H), 3.95 – 3.88 (m,1H), 3.86 (s, 3H), 3.83 (s, 3H), 3.68 (s, 3H), 3.09 – 2.96 (m, 2...
Embodiment 3
[0041] Embodiment 3: synthetic compound 1-3, please refer to Figure 5 and Figure 6
[0042] .
[0043] Arctigenin (50 mg, 0.134 mmol) was dissolved in 2 ml of acetone, potassium carbonate (37 mg, 0.268 mmol) and 3-nitrobromobenzene (33 mg, 0.161 mmol) were added in turn, and refluxed for 4 hours. Remove the solvent under reduced pressure, add 20 mL of water, filter with suction, wash the filter cake several times with 5% potassium bisulfate solution, and recrystallize methanol after vacuum drying to obtain 42 mg of light yellow solid with a yield of 63%. [α] D 20 =-2.1 ( c 1.0, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.85 (d, J = 7.0 Hz, 1H), 7.62(s, 1H), 7.41 (t, J = 8.2 Hz, 1H), 7.21 (dd, J = 8.2, 1.4 Hz, 1H), 6.97 (d, J = 8.0 Hz, 1H), 6.82 (s, 1H), 6.76 (d, J = 8.1 Hz, 1H), 6.71 (d, J = 8.0 Hz,1H), 6.57 (d, J = 8.0 Hz, 1H), 6.53 (s, 1H), 4.19 (dd, J = 8.7, 7.6 Hz, 1H), 3.91 (t, J = 8.4 Hz, 1H), 3.83 (s, 3H), 3.81 (s, 3H), 3.72 (s, 3H), 2.99 (d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com