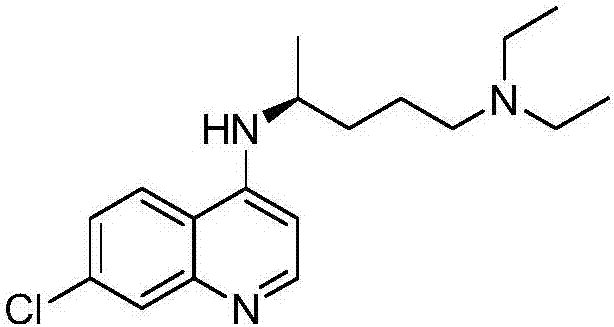
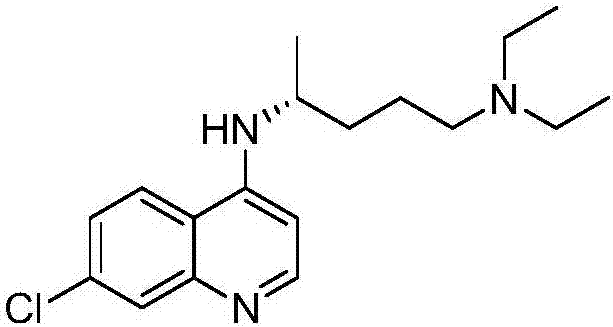
A kind of asymmetric synthetic method of optically pure (r)/(s)-chloroquine
A synthetic method and asymmetric technology, which is applied in the field of asymmetric synthesis of optically pure /-chloroquine, can solve the problems of low total yield and long synthetic route, and achieve the effects of short synthetic route, moderate solubility and moderate reducing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 200mL tetrahydrofuran (solvent), 2.64g (0.12mol) lithium borohydride (reducing agent) and 4.18g (0.012mol) (+)-binaphthol phosphate to a 500mL three-necked flask equipped with a constant pressure funnel and a reflux condenser Esters (chiral reagents), stirred at room temperature for 10 min to obtain a mixed solution, and added 17.8 g (0.1 mol) of 4-amino-7-chloroquinoline, 15.7 g (0.12 mol) of 5-diethylamino-2 - Pentanone and 100 mL of tetrahydrofuran, and slowly drop into the mixed solution from a constant pressure funnel (about 30 min), continue to react at room temperature for 1 h after the drop is completed, and then raise the temperature to 50 ° C and keep the temperature for the reaction. The reaction was detected by TLC. After the reaction was completed (this example required 16 h), it was naturally cooled to room temperature, and 400 mL of saturated saline was added to the reaction system, and then extracted with ethyl acetate, 150 mL each time, 3 times. The...
Embodiment 2
[0029] Reaction process and product treatment are similar to embodiment 1, difference is: solvent, reducing agent and chiral reagent are respectively: 250mL toluene (use 150mL in the three-necked flask, use 100mL in the constant pressure funnel), 11.83g (0.15mol) Potassium cyanoborohydride and 3.5 g (0.015 mol) of (D)-camphorsulfonic acid. 4-Amino-7-chloroquinoline and 5-diethylamino-2-pentanone were slowly dripped into the mixture of reducing agent and chiral reagent from the constant pressure funnel, and then continued to react at room temperature for 2 hours. The holding time is 24h.
[0030] The product is (+)-(S)-chloroquine 14.2g, and the yield is 44%; enantioselectivity is analyzed by chiral HPLC, ee%=81%, [α] 20.5 D =+83.6° (c=1.02, EtOH).
Embodiment 3
[0032] Reaction process and product treatment are similar to embodiment 1, and difference is: solvent, reducing agent and chiral reagent are respectively: 280mL dichloromethane (use 150mL in the three-necked flask, use 130mL in the constant pressure funnel), 42.4g (0.2 mol) sodium triacetoxyborohydride and 2.69 g (0.02 mol) (D)-malic acid. 4-Amino-7-chloroquinoline and 5-diethylamino-2-pentanone are slowly dropped into the mixture of reducing agent and chiral reagent from the constant pressure funnel, and then continue to react at room temperature for 1.5h, and the temperature of the heat preservation reaction is 60°C , holding time 18h.
[0033]The product is (+)-(S)-chloroquine 12.9g, yield rate is 40%; Enantioselectivity is analyzed by chiral HPLC, ee%=77%, [α] 20.5 D =+80.2° (c=1.01, EtOH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com