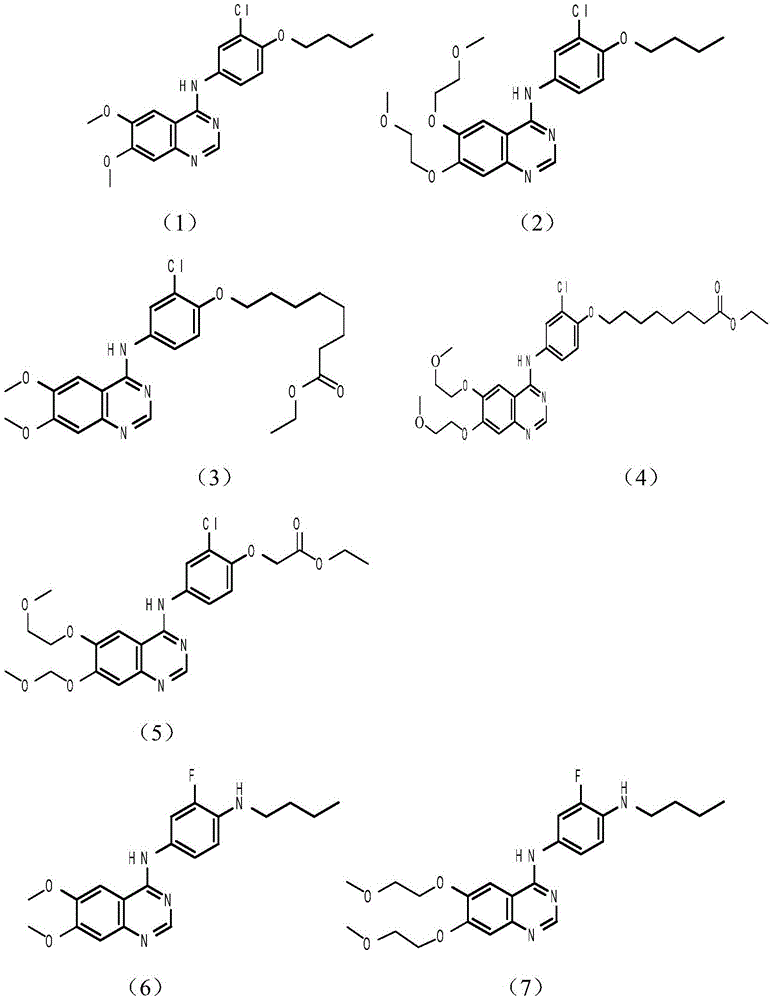
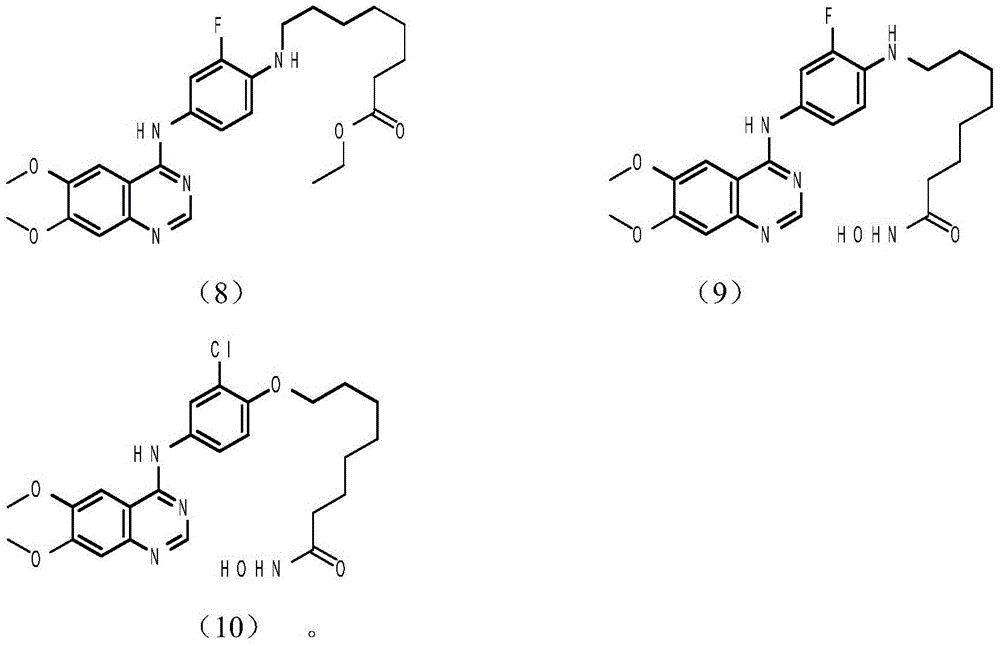
Tyrosine kinase inhibitor, preparation method and application thereof
A tyrosine kinase and inhibitor technology, applied in the field of medicinal chemistry, can solve problems such as poor tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0040] The synthesis of embodiment 13-chloro-4-butoxyaniline
[0041] Phenol potassium salt (0.600g, 2.84mmol), bromo-n-butane (0.501g, 3.70mmol), potassium carbonate (1.96g, 14.2mmol), and 15ml of acetonitrile were added to a three-necked flask and heated to reflux. After the reaction was completed, after a series of The treatment gave 0.540 g of yellow 2-chloro-4-butoxynitrobenzene with a yield of 95.4%. The product obtained by the reaction (0.54, 2.36mmol), iron powder (1.32g, 23.6mmol), ammonium chloride solid (1.62g, 23.6mmol) was added into 15ml of water and methanol mixed solvent into a three-necked flask and heated to reflux. After the reaction was completed, After a series of post-treatments, 0.420 g of the gray product 3-chloro-4-butoxyaniline was obtained, with a yield of 93.0%.
Embodiment 28
[0042] Synthesis of Example 28-(4-amino-2-chlorophenoxy) octanoic acid ethyl ester
[0043]Phenol potassium salt (0.200g, 0.948mmol) and ethyl 8-bromooctanoate (0.308g, 1.23mmol), potassium carbonate (0.601g, 4.34mmol), and 10ml of acetonitrile were added to a three-necked flask and heated to reflux. After the reaction was completed, a series of After post-treatment, 0.28 g of yellow ethyl 8-(2-chloro-4-nitrophenylamino)octanoate was obtained, with a yield of 90.6%. The product (0.28g, 0.816mmol) obtained by the reaction, iron powder (0.457g, 8.16mmol), ammonium chloride solid (0.436g, 8.16mmol) was added into a three-necked flask and heated to reflux with 24ml of water and methanol mixed solvent, after the reaction was completed 0.260 g of the gray product ethyl 8-(4-amino-2-chlorophenoxy)octanoate was obtained through a series of post-treatments, with a yield of 93.5%.
Embodiment 3
[0044] Synthesis of Example 32-(4-amino-2-chlorophenoxy) ethyl acetate
[0045] Phenol potassium salt (0.400g, 1.90mmol), ethyl bromoacetate (0.566g, 2.46mmol), potassium carbonate (1.21g, 8.68mmol), and 15ml of acetonitrile were added into a three-necked flask and heated to reflux for reaction. After the reaction was completed, a series of After post-treatment, 0.46 g of yellow ethyl 2-(2-chloro-4-nitrophenoxy)acetate was obtained, with a yield of 93.9%. The product obtained by the reaction (0.46g, 1.78mmol) and iron powder (0.991g, 17.8mmol), ammonium chloride solid (0.952g, 17.8mmol), 27ml water and methanol mixed solvent were heated to reflux, after the reaction was completed, after After treatment, 0.400 g of ethyl 2-(4-amino-2-chlorophenoxy)acetate was obtained as a gray product, with a yield of 92.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com