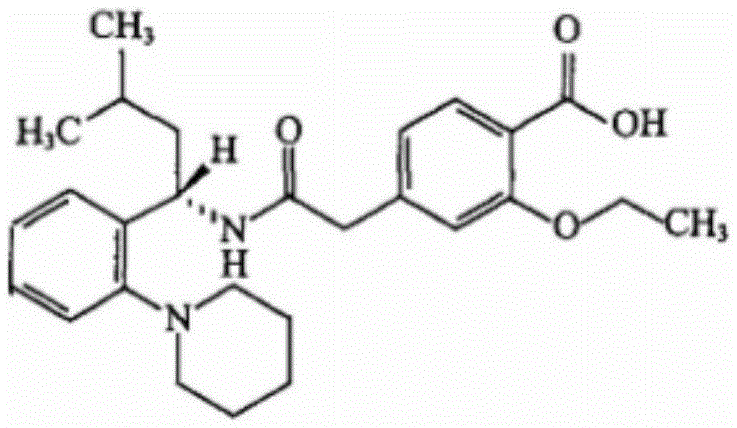
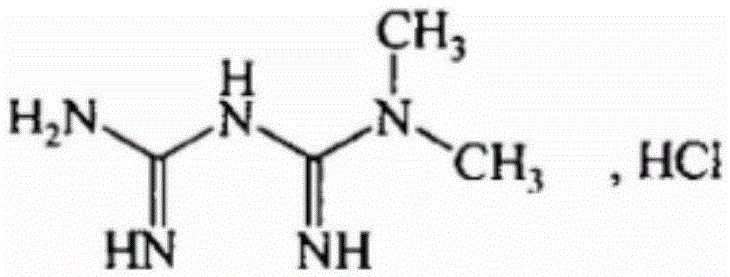
Repaglinide and metformin hydrochloride tablet pharmaceutical composition and preparation method thereof
A technology of metformin tablets and metformin, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve problems such as uneven mixing of low-dose drug repaglinide, and achieve rapid dissolution and reduce exposure , the effect of promoting the efficacy of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
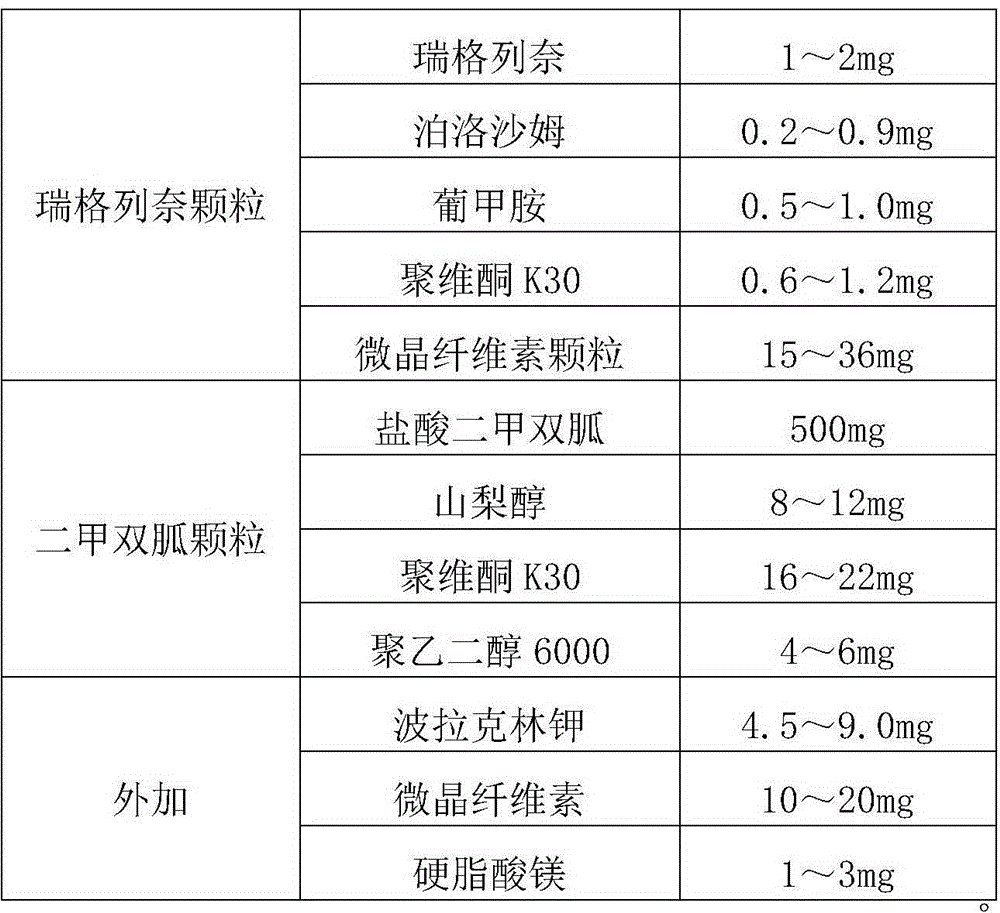
[0046] Embodiment 1: Repaglinide metformin tablet prescription (in 1000 tablets, unit: g):
[0047]
[0048]
[0049] Preparation Process:
[0050] Add 0.2g poloxamer, 0.5g meglumine and 0.6g povidone K30 into an appropriate amount of water, stir to dissolve the materials completely, then slowly add 1g repaglinide, continue stirring to dissolve, and prepare repaglinide solution; take 15g of microcrystalline cellulose particles and place them in the top spray pot of the fluidized bed. 3 / h, the atomization pressure is 1.0Pa, start the liquid supply pump, spray the repaglinide solution into the fluidized bed for granulation, dry after the spraying is completed, and obtain the repaglinide granules; get 500g of metformin hydrochloride, add 16g of povidone K30, 8g of sorbitol, and 4g of polyethylene glycol 6000 were placed in a granulation pot, mixed for 10 minutes, stirred while slowly adding an appropriate amount of water for granulation, and granulated with a sieve plate ...
Embodiment 2
[0051] Embodiment 2: Repaglinide metformin tablet prescription (in 1000 tablets, unit: g):
[0052]
[0053] Preparation Process:
[0054] Add 0.9g poloxamer, 1.0g meglumine and 1.2g povidone K30 into an appropriate amount of water, stir to dissolve the material completely, then slowly add 2g repaglinide, continue stirring to dissolve, and prepare repaglinide solution; take 36g of microcrystalline cellulose particles and place them in the top spray pot of the fluidized bed, set the inlet air temperature at 40-60°C, and the inlet air flow at 60-150 3 / h, the atomization pressure is 1.5Pa, start the liquid supply pump, spray the repaglinide solution into the fluidized bed for granulation, dry after the spraying is completed, and obtain the repaglinide granules; get 500g of metformin hydrochloride, add 22g of povidone K30, 12g of sorbitol, and 6g of polyethylene glycol 6000 were placed in a granulation pot, mixed for 10 minutes, stirred while slowly adding an appropriate amou...
Embodiment 3
[0055] Embodiment 3: Repaglinide metformin tablet prescription (in 1000 tablets, unit: g):
[0056]
[0057] Preparation Process:
[0058] Add 0.9g poloxamer, 1.0g meglumine and 1.2g povidone K30 into an appropriate amount of water, stir to dissolve the materials completely, then slowly add 1g repaglinide, continue stirring to dissolve, and prepare repaglinide solution; take 36g of microcrystalline cellulose particles and place them in the top spray pot of the fluidized bed, set the inlet air temperature at 40-60°C, and the inlet air flow at 60-150 3 / h, the atomization pressure is 2.0Pa, start the liquid supply pump, spray the repaglinide solution into the fluidized bed for granulation, dry after the spraying is completed, and obtain the repaglinide granules; get 500g of metformin hydrochloride, add 20g of povidone K30, 10g of sorbitol, and 5g of polyethylene glycol 6000 were placed in a granulation pot, mixed for 10 minutes, stirred while slowly adding an appropriate amo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com