Leuprolide acetate preparing method
A technology of leuprolide acetate and leuprolide, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as complex production procedures, difficulty in salt conversion, long cycle, etc., and increase time and workload, increase product yield, and reduce the effect of waste liquid discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
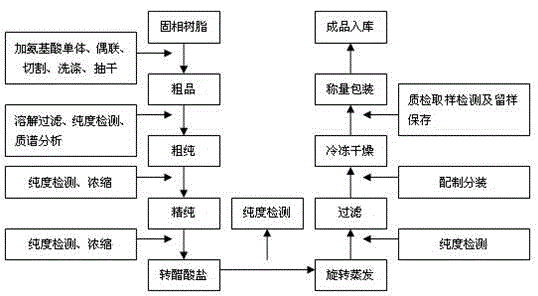
[0028] Embodiment 1: refer to figure 1
[0029] 1. Synthesis of crude leuprolide: solid phase resin Boc-Pro- ( polystyrene resin) placed in the reaction flask, with anhydrous CH 2 Cl 2 Wash 3 times, stir for 10 minutes each time, and drain. Deprotection group: add 9-10NHCl / iPrOH+CH 2 Cl 2 , stirred for 60 minutes, and drained. Washing: CH 2 Cl 2 Wash 3 times and drain; DMF wash 1 time and drain. Neutralization: adding triethylamine / CH 2 Cl 2 Wash 3 times, stir for 5 minutes each time, and drain. Washing: Wash once with DMF and drain; CH 2 Cl 2 Wash 5-6 times until neutral, then drain. Dipeptide connection: Boc-Arg·HClHOBT and DCCI were respectively dissolved in anhydrous DMF and poured into the resin, the mouth of the bottle was tightly sealed, and stirred overnight. The next day, the solvent was drained and washed with CH 2 Cl 2 Wash 3 times, wash 4 times with absolute ethanol, wash 2 times with DMF, CH 2 Cl 2 Wash 3 times and drain. Detection of free a...
Embodiment 2
[0042] Embodiment 2: refer to figure 1
[0043] 1. Synthesis of crude leuprolide: solid phase resin Boc-Pro- ( polystyrene resin) placed in the reaction flask, with anhydrous CH 2 Cl 2 Wash 3 times, stir for 10 minutes each time, and drain. Deprotection group: add 9-10NHCl / iPrOH+CH 2 Cl 2 , stirred for 60 minutes, and drained. Washing: CH 2 Cl 2 Wash 3 times and drain; DMF wash 1 time and drain. Neutralization: adding triethylamine / CH 2 Cl 2 Wash 3 times, stir for 5 minutes each time, and drain. Washing: Wash once with DMF and drain; CH 2 Cl 2 Wash 5-6 times until neutral, then drain. Dipeptide connection: Boc-Arg·HClHOBT and DCCI were respectively dissolved in anhydrous DMF and poured into the resin, the mouth of the bottle was tightly sealed, and stirred overnight. The next day, the solvent was drained and washed with CH 2 Cl 2 Wash 3 times, wash 4 times with absolute ethanol, wash 2 times with DMF, CH 2 Cl 2 Wash 3 times and drain. Detection of free a...
Embodiment 3
[0056] Embodiment 3: refer to figure 1
[0057] 1. Synthesis of crude leuprolide: solid phase resin Boc-Pro- ( polystyrene resin) placed in the reaction flask, with anhydrous CH 2 Cl 2 Wash 3 times, stir for 10 minutes each time, and drain. Deprotection group: add 9-10NHCl / iPrOH+CH 2 Cl 2 , stirred for 60 minutes, and drained. Washing: CH 2 Cl 2 Wash 3 times and drain; DMF wash 1 time and drain. Neutralization: adding triethylamine / CH 2 Cl 2 Wash 3 times, stir for 5 minutes each time, and drain. Washing: Wash once with DMF and drain; CH 2 Cl 2 Wash 5-6 times until neutral, then drain. Dipeptide connection: Boc-Arg·HClHOBT and DCCI were respectively dissolved in anhydrous DMF and poured into the resin, the mouth of the bottle was tightly sealed, and stirred overnight. The next day, the solvent was drained and washed with CH 2 Cl 2 Wash 3 times, wash 4 times with absolute ethanol, wash 2 times with DMF, CH 2 Cl 2 Wash 3 times and drain. Detection of free a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com