Pretreatment solution for electroless plating and electroless plating method
一种前处理液、无电电镀的技术,应用在液态化学镀覆、金属材料涂层工艺、涂层等方向,能够解决限制、处理步骤多、触媒核脱离等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
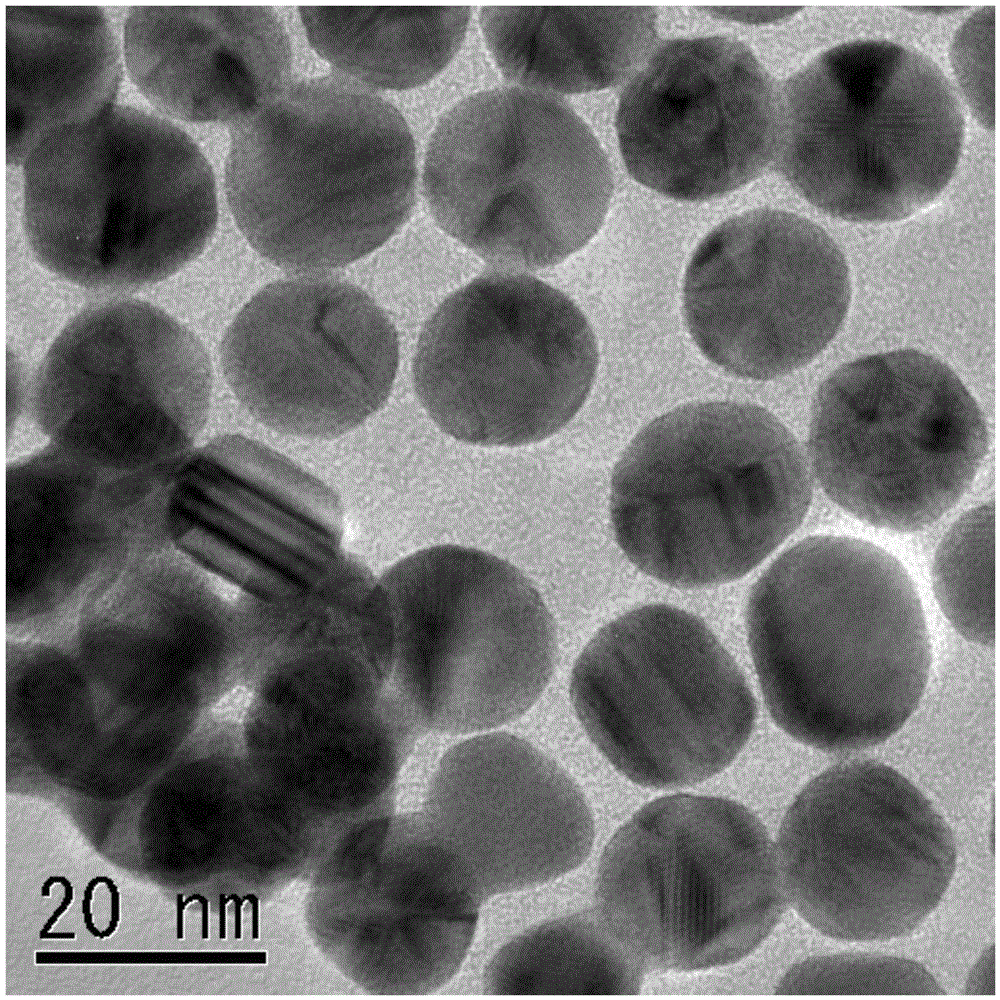
[0059] Dissolve 0.1 g / L sodium tetrachloroaurate (III) tetrahydrate and 1.0 g / L xylitol in an aqueous sodium hydroxide solution (pH = 12 ), and reduced with trisodium citrate dihydrate to obtain gold (Au) colloidal solution. The average diameter of gold (Au) nanoparticles is 20nm, and more than 90% of them are in the range of 10-30nm (d=20±10nm). Gold (Au) nanoparticles with a particle diameter of 20 nm were observed with a transmission electron microscope (JEM-2010 manufactured by JEOL Ltd.). figure 1 A transmission electron microscope image is shown. It can be clearly seen from this figure that on the surface of the gold (Au) nanoparticles, the phycoclusters are automatically aligned at equal intervals with a size close to the atomic level of gold (Au).
[0060] Next, the obtained gold (Au) colloidal solution was dispersed in 80°C aqueous solution of 1 prescribed hydrochloric acid, sulfuric acid and potassium hydroxide, and similarly observed with a transmission electron m...
Embodiment 2
[0062] In the same manner as in Example 1, the gold (Au) conversion concentration of sodium tetrachloroaurate (III) tetrahydrate was changed to 1 g / L, 5 g / L and 9 g / L, and the concentration of xylitol was changed to Change to 15g / L, 0.5g / L and 150g / L. The particle diameters of the obtained gold (Au) nanoparticles are respectively d=20±10nm, d=30±10nm and d=50 with respect to the gold (Au) conversion concentration of 1g / L, 5g / L and 9g / L ±20nm.
Embodiment 3
[0064] Use mannitol, glycerol or erythritol instead of xylitol to carry out the same experiment as in Example 1, and obtain gold (Au) colloids of d=20±10nm, d=20±10nm and d=20±10nm respectively Nanoparticles. In the same manner as in Example 1, the resulting gold (Au) colloidal solution was dispersed in an 80°C aqueous solution of 1 regulated hydrochloric acid, sulfuric acid and potassium hydroxide, and in the same manner as in Example 1, no gold (Au) nanoparticles were observed. The surface properties of the particles change.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com