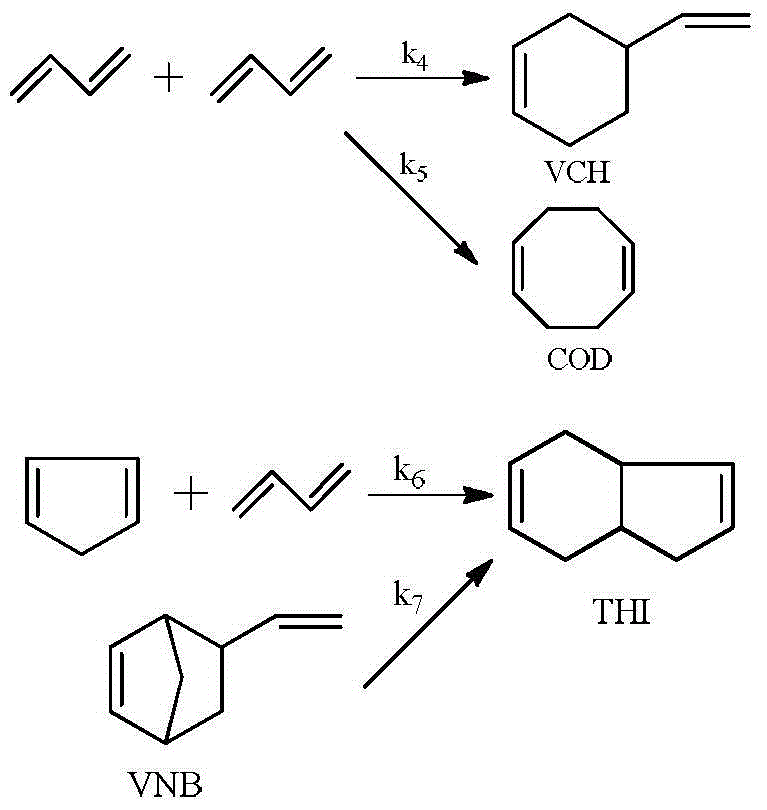
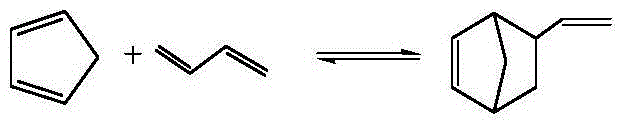Synthetic method of vinyl norbornene
A technology for vinyl norbornene and a synthesis method, which is applied to chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve problems such as many side reactions, reduce production costs, avoid the generation of reaction hot spots, and is beneficial to sealing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
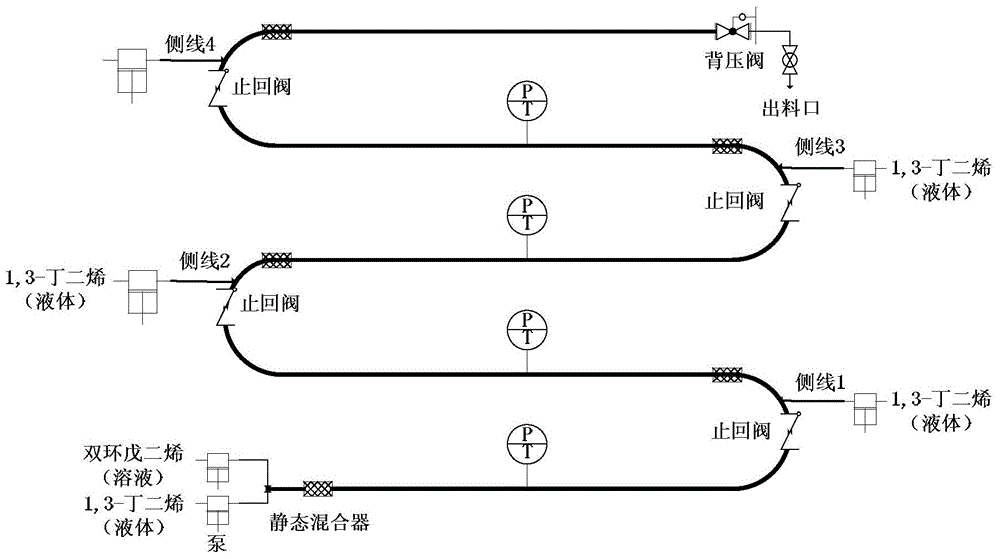
[0033] A cyclopentadiene-containing dicyclopentadiene toluene solution with a mass fraction of 60 wt% was prepared, wherein cyclopentadiene in the dicyclopentadiene accounted for 100 wt%. Under normal temperature conditions, the dicyclopentadiene toluene solution with high concentration of cyclopentadiene is directly transported from the main line feed port to the tubular reaction device, and 1,3-butadiene is fed from the main line feed port and the first side line Inlet and the second side line feed port are divided into three equal parts and sent to the tubular reaction device, the total mass flow rate of 1,3-butadiene is 20.5g / min, and the dicyclopentadiene toluene solution with high concentration cyclopentadiene The mass flow rate is 210.0g / min, and the molar ratio of 1,3-butadiene to high-concentration cyclopentadiene dicyclopentadiene (in terms of moles converted into cyclopentadiene, the same below) is 0.2.1 , The two streams of 3-butadiene and dicyclopentadiene are mix...
Embodiment 2
[0035]A cyclopentadiene-containing dicyclopentadiene toluene solution with a mass fraction of 60 wt % was prepared, wherein cyclopentadiene in dicyclopentadiene accounted for 80 wt %. Under normal temperature conditions, the dicyclopentadiene toluene solution with high concentration of cyclopentadiene is directly transported from the main line feed port to the tubular reaction device, and 1,3-butadiene is fed from the main line feed port and the first side line Inlet and the second side line feed port are transported to the tubular reaction device in equal amounts, the total mass flow rate of 1,3-butadiene is 35.7g / min, and the mass of dicyclopentadiene toluene solution with high concentration cyclopentadiene The flow rate is 182.0g / min, and the molar ratio of 1,3-butadiene to high-concentration cyclopentadiene dicyclopentadiene is 0.4. The two streams of 1,3-butadiene and dicyclopentadiene are mixed in a meridian state After being mixed with the reactor, it enters the reactio...
Embodiment 3
[0037] Dicyclopentadiene containing cyclopentadiene with a mass fraction of 100 wt% is used as a raw material, wherein cyclopentadiene accounts for 50 wt% of the dicyclopentadiene. Under normal temperature conditions, dicyclopentadiene with high concentration of cyclopentadiene is directly transported from the main line feed port to the tubular reaction device, and 1,3-butadiene is fed from the main line feed port, the first side line feed port and The second side line feed port is delivered to the tubular reaction device in three equal quantities. The total 1,3-butadiene mass flow rate is 30.3g / min, and the dicyclopentadiene toluene solution mass flow rate of high-concentration cyclopentadiene is 37.0g / min, the molar ratio of 1,3-butadiene to high-concentration cyclopentadiene dicyclopentadiene is 1.0. The two streams of 1,3-butadiene and dicyclopentadiene are mixed by a static mixer After entering the reaction section, the reaction takes place in the tubular reactor. The re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com