Preparation method of CSF (Classical Swine Fever) and PR (Pseudorabies) bivalent live vaccine and product of CSF and PR bivalent live vaccine
A technology of pseudorabies and pseudorabies virus, applied in the field of veterinary biological products, can solve the problems of difficulty in rationally arranging various immunization procedures, affecting the prevention effect, increasing costs, etc., to achieve uniform and stable product quality, improve vaccine production and quality, cost-increasing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 swine fever, pseudorabies dual live vaccine
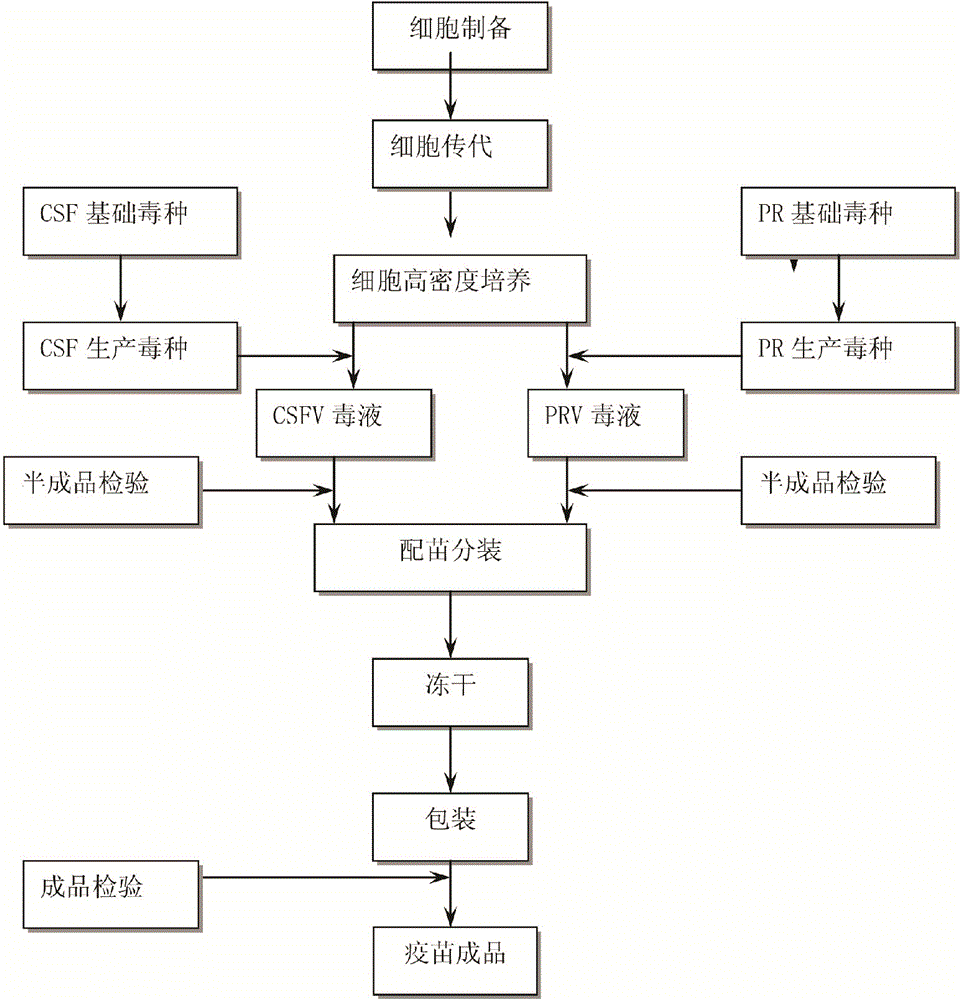
[0035] Such as figure 1 Shown, the preparation method of swine fever, pseudorabies dual live vaccine, technical scheme is:
[0036] (1) Culture method: use bioreactor or cell roller bottle to cultivate cells and propagate classical swine fever virus and pseudorabies virus.
[0037] (2) Select susceptible cells as cells for seedling production: one of bovine testis cells, chicken embryo fibroblasts, porcine testis (ST) passage cells, pig kidney (PK15) passage cells, and pig kidney (IBRS-2) passage cells .
[0038] (3) Passage and culture of cells for seedling production: the above-mentioned susceptible cells are digested and passed by EDTA-trypsin cell dispersion liquid, and continued to be cultured with cell growth liquid. When a good monolayer is formed, they are used for continued passage or virus inoculation;
[0039] (4) Propagation of poisonous species for production:
[0040] A. Pro...
Embodiment 2
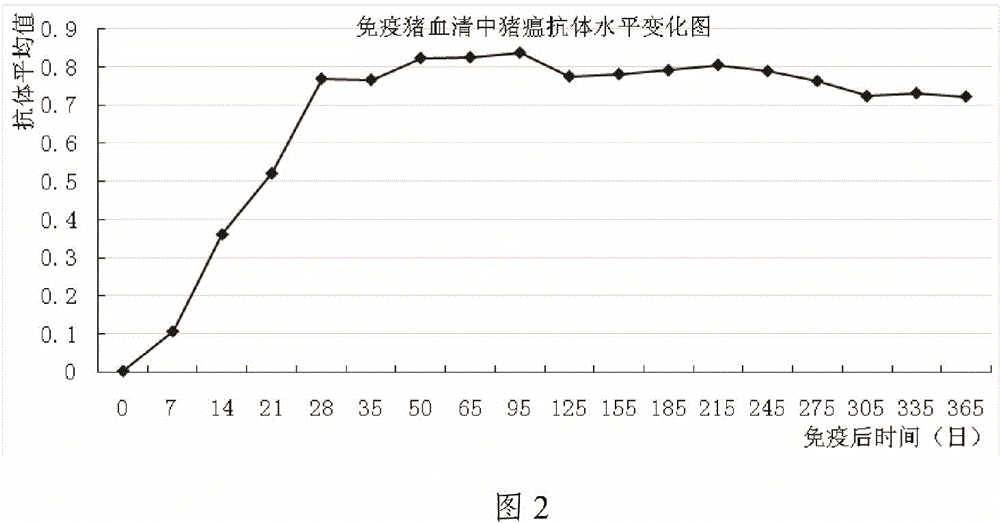
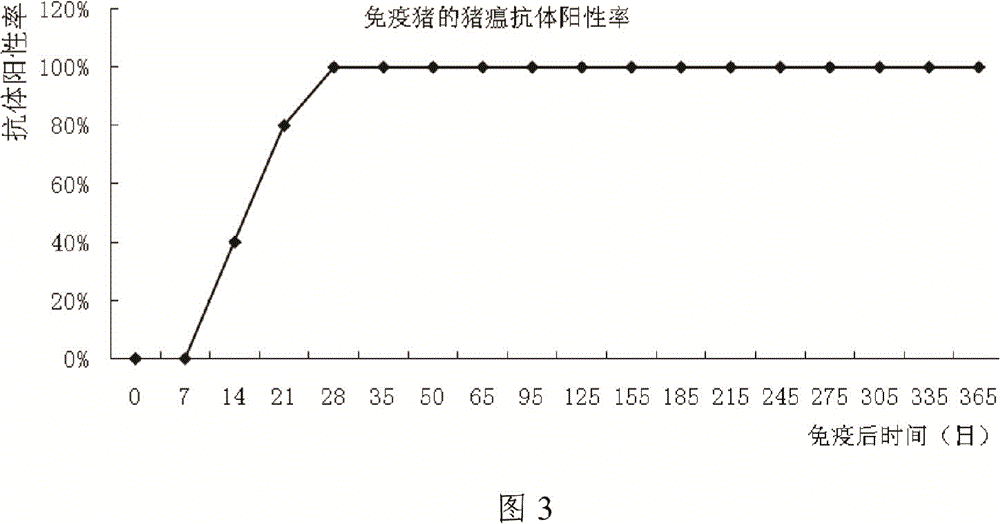
[0087] The immune test of embodiment 2 swine fever, pseudorabies dual live vaccine to pig
[0088] 1 Materials and methods
[0089] 1.1 Materials
[0090] 1.1.1 The live vaccine of swine fever and pseudorabies is produced by Guangdong Yongshun Biopharmaceutical Co., Ltd. and passed the inspection.
[0091] 1.1.2 The pigs used in the test are from the binary hybrid pigs self-propagating and self-raised in the experimental animal farm of Guangdong Yongshun Biopharmaceutical Co., Ltd., and have no history of CSFV, PRRSV, PRV, PPV, PCV-Ⅱ, and have never been vaccinated against CSF vaccine or PR vaccine. and 20 healthy pigs with PRRS vaccine. After detection by ELISA and neutralization test methods, no neutralizing antibodies to swine fever and pseudorabies were selected.
[0092]1.1.3 The swine fever antibody detection kit was purchased from IDEXX company.
[0093] 1.1.4 A pseudorabies antibody detection kit was purchased from IDEXX Company.
[0094] 1.2 Method
[0095] 1.2....
Embodiment 3
[0109] Comparative experiment of embodiment 3 swine fever, pseudorabies dual live vaccine and swine fever live vaccine, pseudorabies live vaccine
[0110] 1 material
[0111] 1.1 3 batches of live vaccines for swine fever and pseudorabies used in the test, batch numbers: CSF-PR200801, CSF-PR200802, CSF-PR200803, passed the inspection according to the current "Chinese Veterinary Pharmacopoeia" inspection method;
[0112] Three batches of live swine fever vaccines, batch numbers: CSF200801, CSF200802, and CSF200803, passed the inspection according to the current "Chinese Veterinary Pharmacopoeia" "Swine Fever Live Vaccine (Cell Source)" inspection method;
[0113] Three batches of live pseudorabies vaccine, batch numbers: PR200801, PR200802, PR200803, passed the test according to the current "Chinese Veterinary Pharmacopoeia" "Pseudorabies Live Vaccine".
[0114] 1.2 Experimental animals
[0115] 1.2.1 Rabbits come from New Zealand big-eared whites that are self-propagating an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com