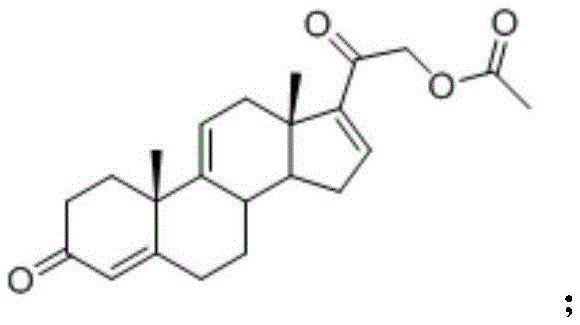
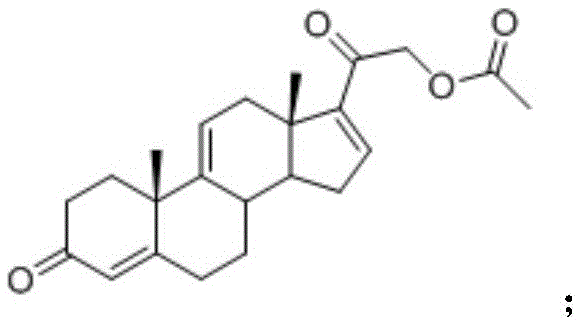
Method for preparing steroidanti-inflammatory drugintermediatetetraene acetate using enzyme process
A technology for the preparation of tetraenyl acetate and enzymatic method, which is applied in the field of tetraenyl acetate, an intermediate of steroid anti-inflammatory drugs, can solve the problems of low concentration of reaction substrate, complicated product separation, unfriendly environment, etc., and achieve production Low cost, simple preparation steps, and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Screening of steroidal 1,2 dehydrogenase
[0024] Add 10 mg of compound II to 2 mL of pH 7.0 phosphate buffer solution, then add 10 mg of steroid 1,2 dehydrogenase of different brands purchased from Suzhou Hanzyme Biotechnology Co., Ltd., shake at 30 °C for 2 h, and detect The conversion rate of the substrate is shown in Table 1.
[0025] Table 1
[0026] enzyme
[0027] It can be seen from Table 1 that, under other conditions being the same, when the steroidal 1,2 dehydrogenase with brand name EW1145 is used as the steroidal 1,2 dehydrogenase, the conversion rate of the substrate is the highest.
Embodiment 2
[0028] Substrate conversion rate situation under the different temperature conditions of embodiment 2
[0029] Add 10 mg of compound II to 2 mL of pH 7.0 phosphate buffer solution, add 1 mg of steroidal 1,2 dehydrogenase (purchased from Suzhou Hanzyme Biotechnology Co., Ltd., brand EW1145, the same below), under different temperature conditions , shaken for 12 hours, and the conversion rate of the detected substrate is shown in Table 2.
[0030] Table 2
[0031] temperature °C
[0032] It can be seen from Table 2 that, under other conditions being the same, the conversion rate of compound II is the highest when the reaction temperature is controlled at 30°C.
Embodiment 3
[0033] Substrate conversion rate situation under the different pH conditions of embodiment 3
[0034] Add 10mg of compound II to 2mL of phosphate buffer solution with different pH, add 1mg of steroidal 1,2 dehydrogenase, shake and react at 30°C for 12h, and detect the conversion rate of the substrate as shown in Table 3.
[0035] table 3
[0036] pH
[0037] As can be seen from Table 3, under other conditions being the same, the conversion rate of the substrate is the highest when the above reaction is carried out in a buffer solution with a pH of 7.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com