Synthesis method of high-purity o-bromoacetophnones
A synthesis method and technology of acetophenone, applied in the field of synthesis of high-purity o-bromoacetophenone, can solve the problems of expensive raw materials, lack of production conditions, low purity, etc., to achieve industrial production, improve production operation environment, shorten The effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
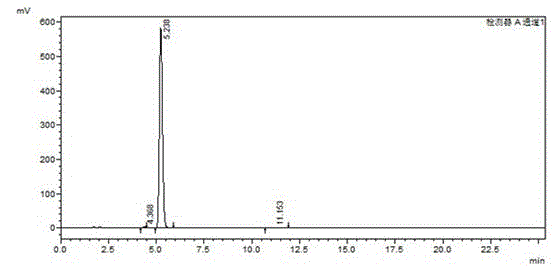
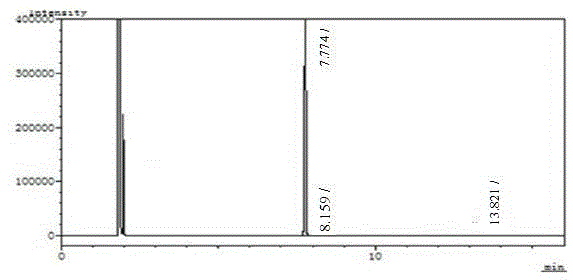
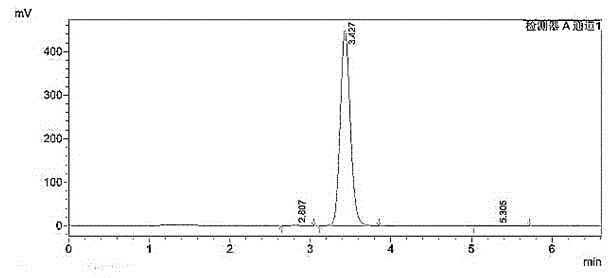
Image
Examples
preparation example Construction
[0016] The synthesis method of high-purity o-bromoacetophenone of the present invention comprises the following steps: add methylmagnesium bromide Grignard reagent in a container, under the normal temperature condition, drop the tetrahydrofuran solution of o-bromoxynil, after adding, there will be a large amount of A white solid appears; when there is no raw material detected by HPLC, stop the reaction; add it dropwise to hydrochloric acid solution, separate liquid, spin dry, and distill under reduced pressure to obtain a colorless liquid product (high-purity o-bromoacetophenone product). The molar ratio of o-bromoxynil and methylmagnesium bromide is 1:1.05.
[0017] The detailed steps of the present invention are as follows: Among them, the dosage is as follows: o-bromoxynil: 91g, methylmagnesium bromide (2M): 262.5ml, THF (Tetrahydrofuran, tetrahydrofuran): 90ml, concentrated hydrochloric acid: 60ml, toluene: 300ml. Add 262.5ml of methylmagnesium bromide (2M) into a 500ml re...
example 2
[0022] Example 2: The dosage is as follows: o-bromoxynil: 91g, methylmagnesium bromide (2M): 275ml, THF (Tetrahydrofuran, tetrahydrofuran): 90ml, concentrated hydrochloric acid: 70ml, toluene: 300ml.
[0023] Add 275ml (0.55) of methylmagnesium bromide (2M) into a 500ml reaction bottle, control the reaction temperature at 20-25°C, add dropwise 91g (0.5mol) o-bromoxynil and 90ml THF solution, control the reaction temperature at 20-25°C , The dropwise addition was completed in about 2 hours, and a large amount of white solid appeared after the addition. Reaction was continued at this temperature for 1 hour. Use HPLC to monitor the reaction situation, when the raw materials of the reaction solution disappear, stop the reaction, add the reaction solution dropwise to 70ml concentrated hydrochloric acid and 300ml water, separate the liquids, extract the aqueous phase with 300ml toluene, combine the organic phases, wash with 200ml water, and decompress -0.095MPa Concentrate until no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com