Assays and methods for selecting a treatment regimen for a subject with depression
A depression and individual technology, applied in the direction of microbial determination/testing, biochemical equipment and methods, special data processing applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0543] Example 1. Identification of Biomarkers for Selecting Depressed Patients for Treatment with Folate-Containing Compounds in Combination with SSRIs
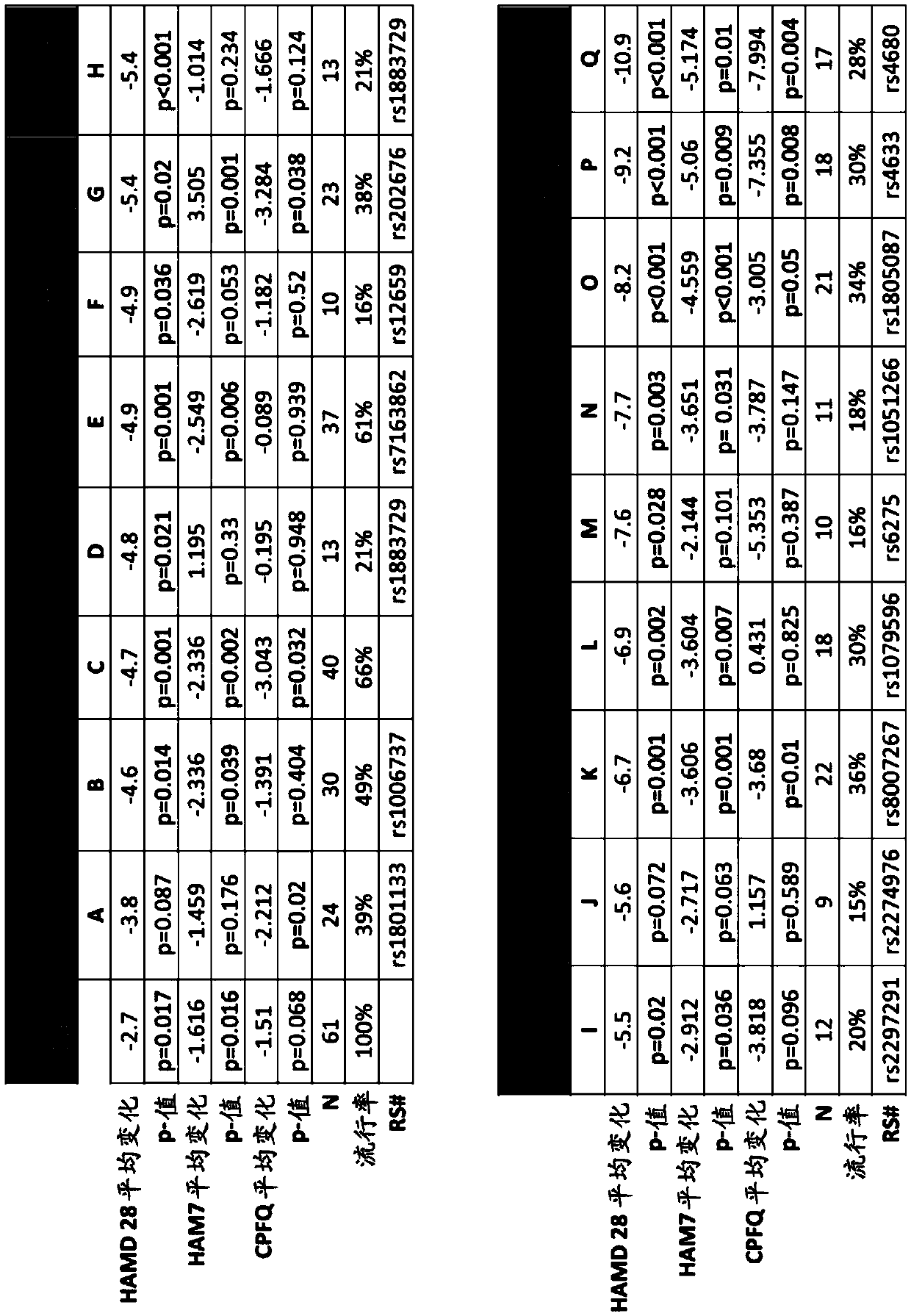
[0544] A double-blind, placebo-controlled study of 6(S)-5-MTHF in SSRI-resistant outpatients with major depressive disorder (MDD) to identify specific biomarker combinations that Greater efficacy response (e.g., a response greater than the additive response produced by the two SNPs, or greater than that produced by either SNP alone) when the patient is administered a folate-containing compound (e.g., 6(S)-5-MTHF) in addition to a depressant Responses to Responses) Correlation. See eg, Papakostas GI et al., Am J Psychiatry, 169:1267-74 (2012).
[0545] Multiple genetic biomarkers, one genetic biomarker, and clinical features were assessed for their impact on the efficacy of treatments containing folate-containing compounds (such as 6(S)-5-MTHF) and SSRIs in patients with depression. kind of combination. Specifically, the s...
Embodiment 2
[0556] Example 2. An Exemplary Method for Selecting Patients for Folate-Containing Boost Therapy (with SSRIs) and Individualized Prognosis of MDD Patients Undergoing Such Treatment
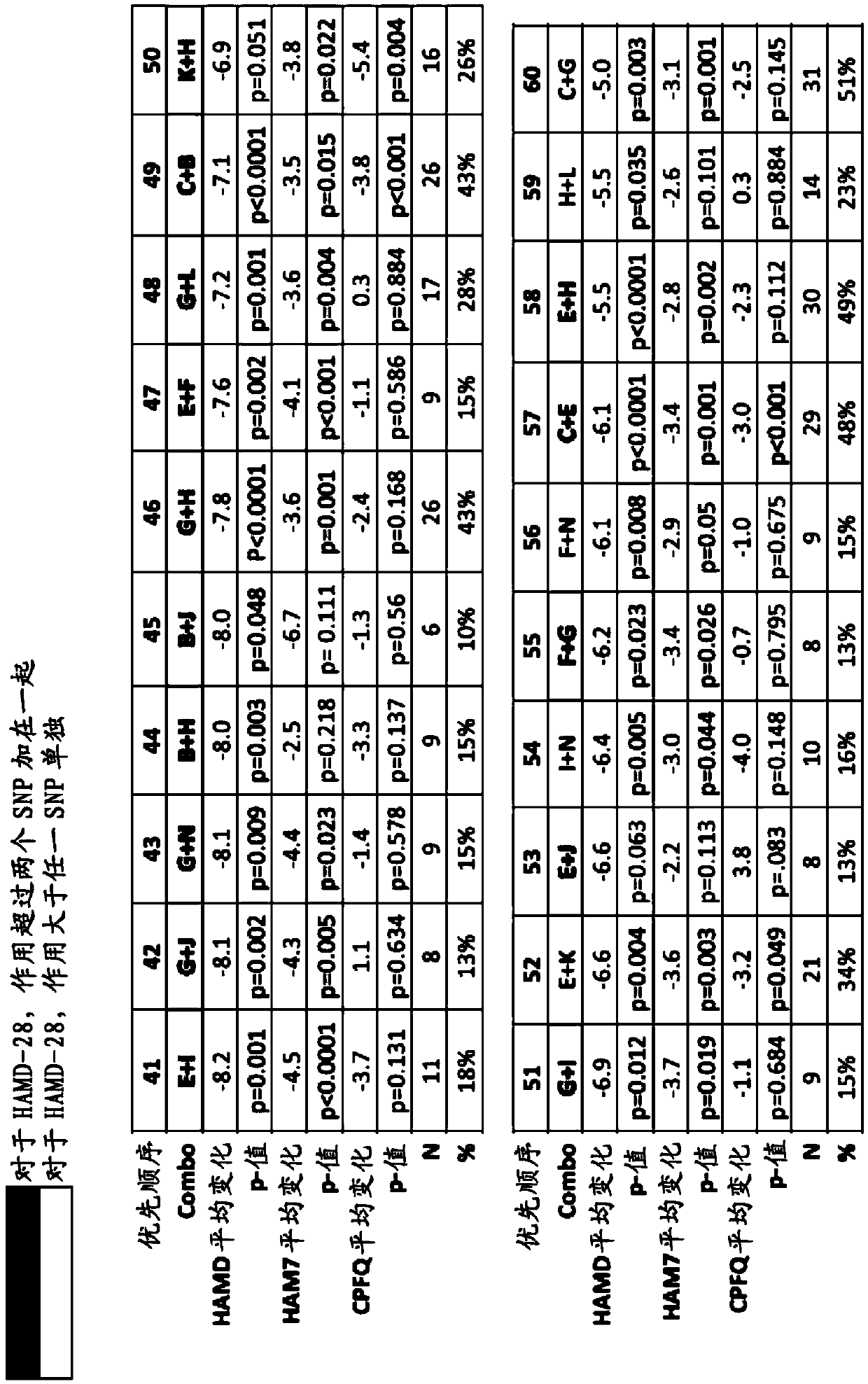
[0557] Based on the use of folate-containing compounds such as A double-blind, placebo-controlled, multi-site study of MDD treatment augmentation (in which 36 patients received folic acid-containing compounds such as ), whose HAMD-28 has a decrease from the baseline measurement), patients diagnosed with at least one of the conditions described in the test series (PT) (with corresponding "values" (as shown in detail later)) generally follow the following Expected HAMD-28 reduction responses shown in the table.
[0558] Accordingly, also provided herein are methods of treating a patient with MDD and / or determining or improving the effectiveness of antidepressants taken by a patient with MDD. For example, in some embodiments, the method may comprise (1) screening for treatment-resistant MDD patient...
Embodiment 3
[0571] Example 3. Effect of Adjunctive L-Methylfolate 15 mg in Depressed Patients Stratified by Biomarker Level and Genotype: Results from a Randomized Clinical Trial
[0572] This example illustrates methods for improving the effectiveness of antidepressants, such as selective serotonin reuptake inhibitors, administered to human patients diagnosed with or at risk of developing depression. The method comprises determining whether the patient carries at least one single nucleotide polymorphism (SNP), wherein the SNP is associated with an increased effectiveness of the antidepressant when administered in combination with a folate-containing compound (e.g., L-methylfolate) relevant. This embodiment also provides for use in the treatment of at least one symptom of depression (e.g., depressed mood, guilt, decreased work or interest, psychomotor retardation, agitation, psychogenic anxiety, physical anxiety, systemic symptoms, cognitive obstacles or any combination thereof). The me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com