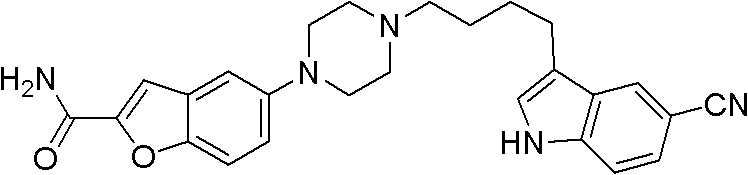
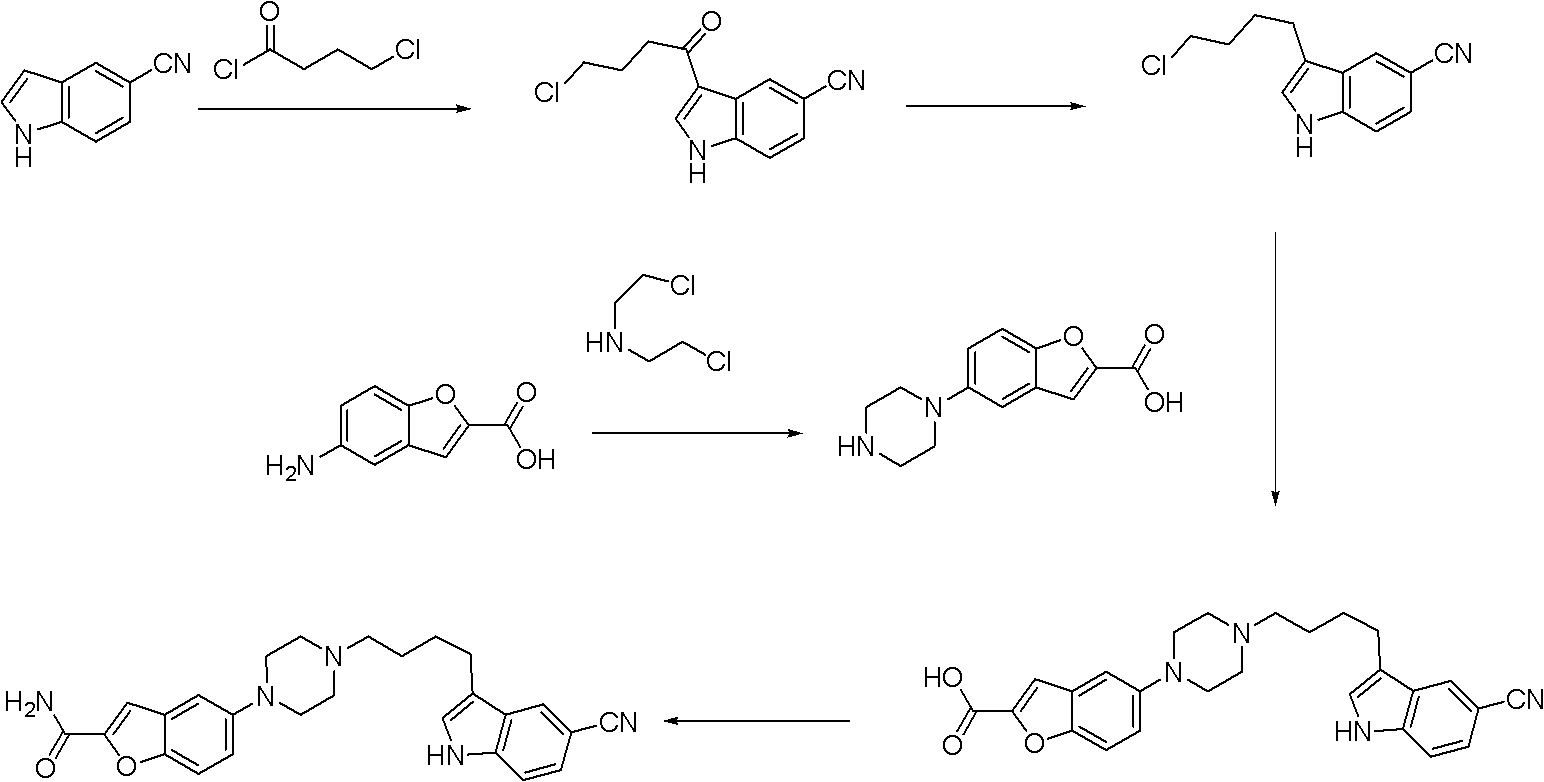
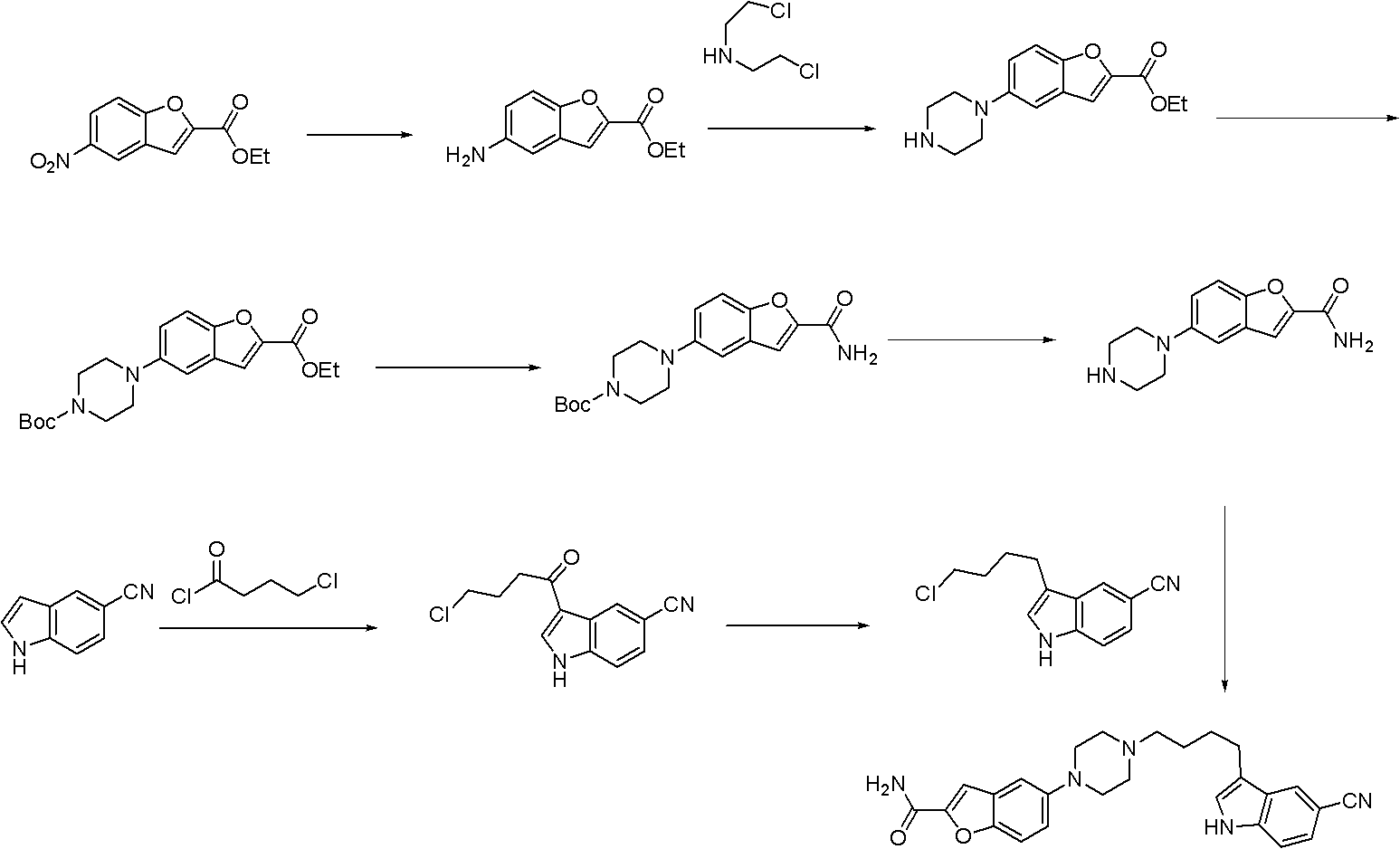
Synthesis method for antidepressant drug vilazodone
A technology for a drug vilazodone and a synthesis method, which is applied in the field of drug synthesis and can solve problems such as increasing the difficulty of purification, reducing reaction steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The first step: the preparation of intermediate 5-nitrobenzofuran-2-carboxylic acid
[0035]
[0036] Add 30g of 5-nitrosalicylaldehyde in a 1L three-necked flask, dissolve it with 170mL of N,N-dimethylformamide, then add 49.6g of potassium carbonate and 29.9g of ethyl bromoacetate in sequence, and the temperature of the reaction solution is raised to 90 ° C, magnetic stirring reaction. After TLC detection of 5-nitrosalicylaldehyde disappearance, 30 g of water was added, and the stirring reaction at 90° C. was continued. Cool the reaction solution after the TLC detection detection reaction is completed. Pour the cooled reaction liquid into 1.8 L of 1 mol / L dilute hydrochloric acid, stir for half an hour and then filter, recrystallize the obtained solid with 30 mL of methanol and 150 mL of ethyl acetate, and filter to obtain 31.7 g of the product, with a yield of 85%
[0037] The second step: the preparation of intermediate 5-nitrobenzofuran-2-carboxamide
[0038] ...
Embodiment 2
[0050] This embodiment is the same as Embodiment 1, the difference is the following steps:
[0051] The first step: the preparation of intermediate 5-nitrobenzofuran-2-carboxylic acid
[0052]
[0053] Add 30g of 5-nitrosalicylaldehyde in a 1L three-necked flask, dissolve it with 170mL of N,N-dimethylformamide, then add 54.5g of potassium carbonate and 59.8g of ethyl bromoacetate in sequence, and the temperature of the reaction solution is raised to 130°C, magnetic stirring reaction. After TLC detection of 5-nitrosalicylaldehyde disappearance, 30 g of water was added, and the stirring reaction at 90° C. was continued. Cool the reaction solution after the TLC detection detection reaction is completed. Pour the cooled reaction solution into 1.8 L of 1 mol / L dilute hydrochloric acid, stir for half an hour and then filter, recrystallize the obtained solid with 30 mL of methanol and 150 mL of ethyl acetate, and filter to obtain 29.8 g of the product, with a yield of 80%
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com