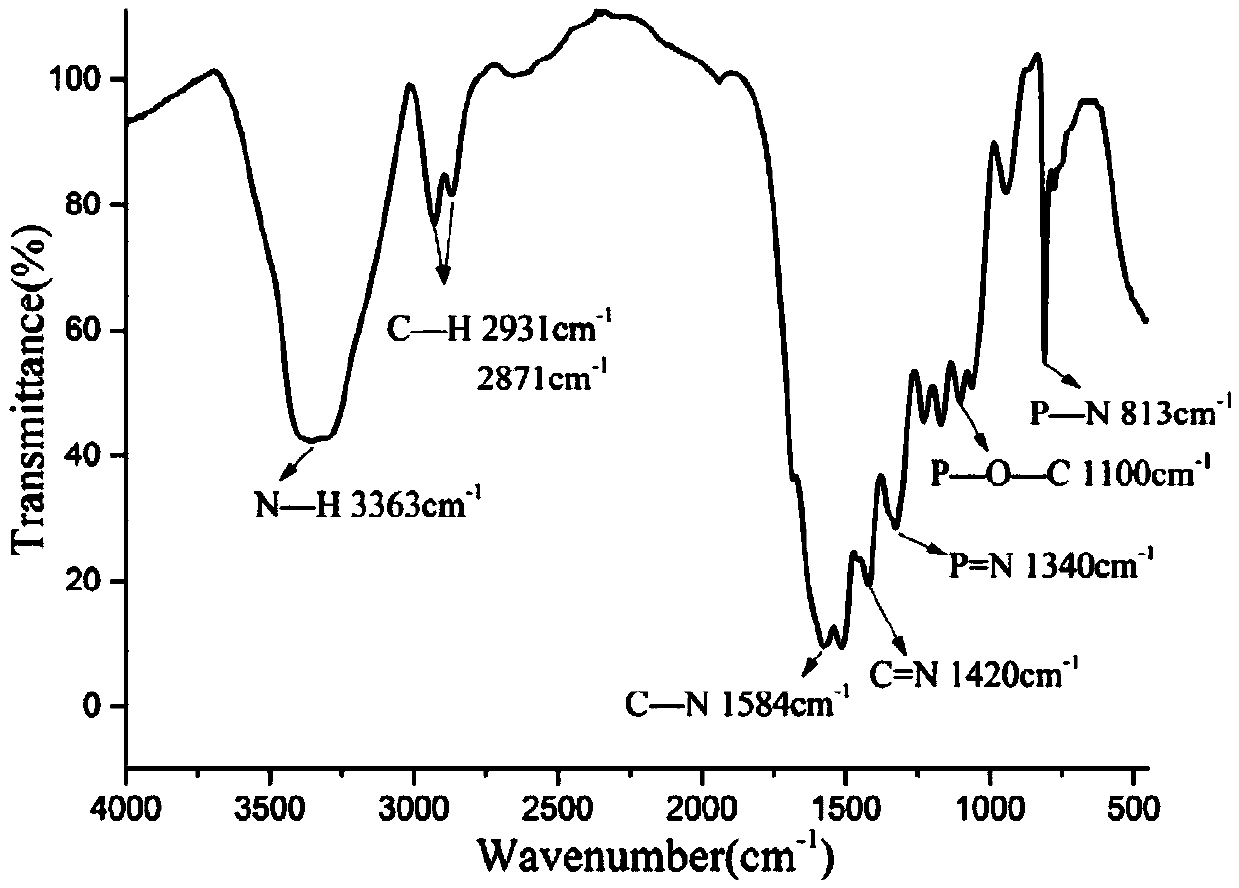
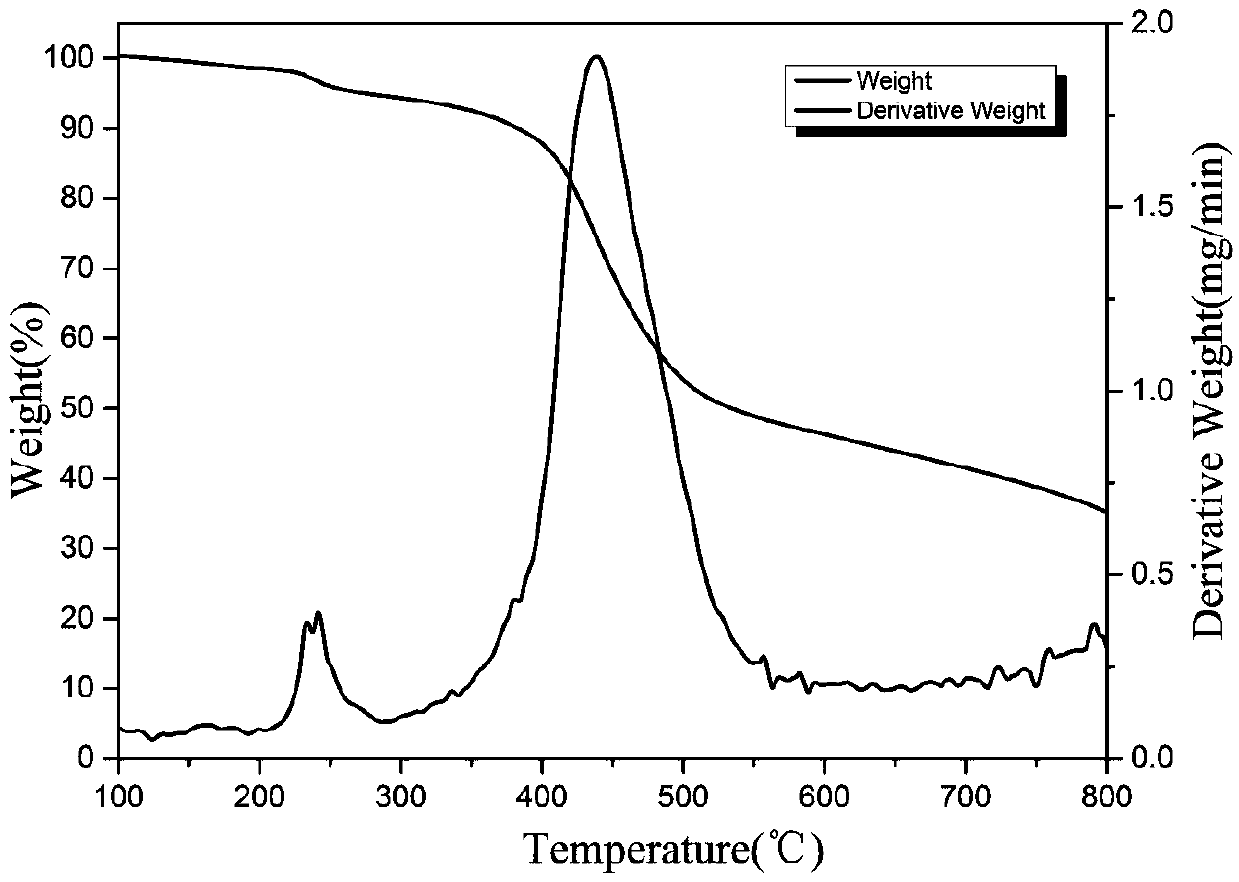
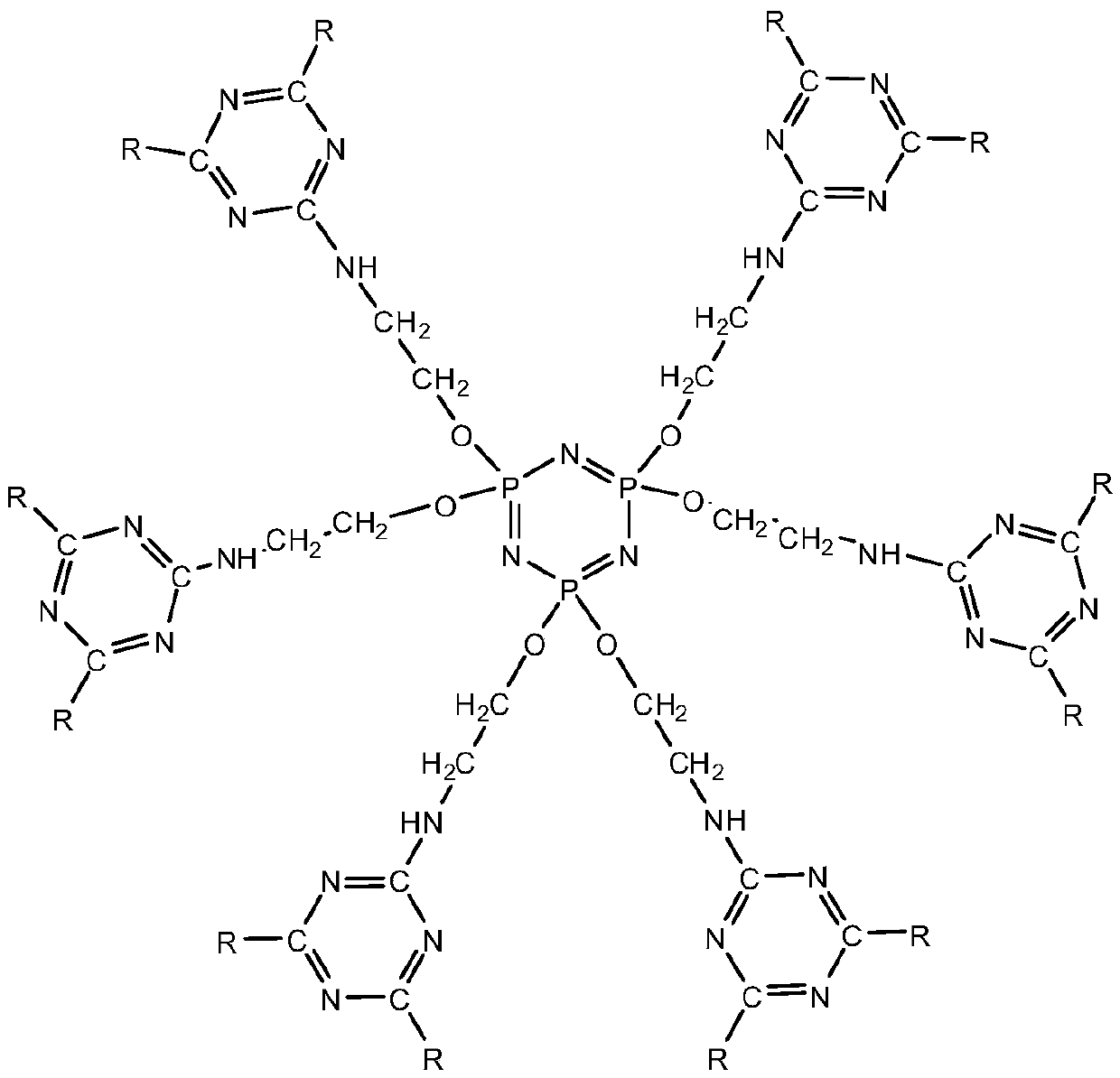
Double base compound based on phosphonitrile and triazine group and preparation method of double base compound
A technology of triazine group and dihydroxy compound, which is applied in the fields of flame retardants and their preparation, double-radical compounds based on phosphazene and triazine groups and their preparation, achieving high char formation rate, good processability, thermal good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step (1). Dissolve 2.00g cyanuric chloride in 15ml tetrahydrofuran solution, join in the tetrahydrofuran solution 40ml that is dissolved with 0.68g ethanolamine, add sodium carbonate 1.15g, in N 2 In the atmosphere, stir at -15°C for 2 hours to obtain 2,4-dichloro-6-hydroxyethylamino-1,3,5-triazine compound;
[0034] Step (2). Add 0.68g of hexachlorocyclotriphosphazene in 30ml of tetrahydrofuran to the 2,4-dichloro-6-hydroxyethylamino-1,3,5-triazine compound, add 1.15g of sodium carbonate, and heat at 50°C Condensation and reflux under stirring conditions for 5 hours;
[0035] Step (3). Add 1.30 g of ethylenediamine in 10 ml of tetrahydrofuran to the reaction solution; raise the temperature to 40° C., react under stirring and condensation conditions for 3 hours, continue to raise the temperature to 80° C., and react under stirring and condensation conditions 3 hours;
[0036] Step (4). After the reaction, suction filtration, water washing, and drying are performed to ...
Embodiment 2
[0039] Step (1). 4.00g cyanuric chloride is dissolved in 30ml acetone solution, joins in the acetone solution 50ml of 1.36g ethanolamine, adds potassium hydroxide 1.22g, in N 2 In the atmosphere, stir at -10°C for 2 hours to obtain 2,4-dichloro-6-hydroxyethylamino-1,3,5-triazine compound;
[0040] Step (2). Add 1.36g of hexachlorocyclotriphosphazene in 40ml of acetone solution to 2,4-dichloro-6-hydroxyethylamino-1,3,5-triazine compound, add 1.22.00g of potassium hydroxide, Condensation and reflux for 5 hours under stirring at 50°C;
[0041] Step (3). Add 2.80 g of hexamethylenediamine in 15 ml of acetone solution to the reaction solution; raise the temperature to 45° C., react under stirring and condensation conditions for 3 hours, continue to raise the temperature to 85° C., and react under stirring and condensation conditions 3 hours;
[0042] Step (4). After the reaction, suction filtration, water washing, and drying are performed to obtain a diradical compound based on p...
Embodiment 3
[0043] Step (1). 6.00g cyanuric chloride is dissolved in 40ml ethyl acetate solution, joins in the ethyl acetate solution 60ml that is dissolved with 2.05g ethanolamine, adds sodium hydroxide 1.30g, in N 2 In the atmosphere, stir at -5°C for 2.5 hours to obtain 2,4-dichloro-6-hydroxyethylamino-1,3,5-triazine compound;
[0044] Step (2). Add 2.05g of hexachlorocyclotriphosphazene in 50m ethyl acetate solution to 2,4-dichloro-6-hydroxyethylamino-1,3,5-triazine compound, add 1.30g of sodium hydroxide , condensed and refluxed for 5.5 hours under stirring at 55°C;
[0045] Step (3). Add 6.90 g of p-phenylenediamine in 25 ml of ethyl acetate solution to the reaction solution; raise the temperature to 55 ° C, stir and condense for 3 hours, continue to raise the temperature to 85 ° C, stir and condense Under conditions, react for 3 hours;
[0046] Step (4). After the reaction, suction filtration, water washing, and drying are performed to obtain a diradical compound based on phosphaze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com