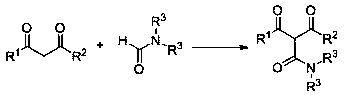
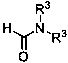
A kind of preparation method of 2-carbamoyl-1,3-dicarbonyl derivative
A technology of carbamoyl and derivatives, which is applied in the field of preparation of organic compounds, can solve the problems of difficult to obtain raw materials, easy to react with water, and high toxicity of acid chlorides, and achieve short reaction time, simple post-treatment, and waste reduction. the effect produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of 2-N,N-dimethylcarbamoyl-1,3-diphenyl-1,3-propanedione
[0038] Using 1,3-diphenyl-1,3-propanedione and N,N-dimethylformamide as raw materials, the reaction steps are as follows:
[0039] Add 1,3-diphenyl-1,3-propanedione (0.224 g, 1 mmol), N,N-dimethylformamide (2 mL), cuprous chloride (0.010 g, 0.1 mmol) and tert-butyl hydroperoxide (0.180 g, 2 mmol), react at 100°C;
[0040] TLC tracking reaction until complete completion;
[0041] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 93%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.00 – 7.90 (m, 2H),7.74 – 7.66 (m, 2H), 7.56 – 7.48 (m, 1H), 7.47 – 7.38 (m, 5H), 7.15 (s, 1H),3.08 (s, 3H), 2.98 (s, 3H).
Embodiment 2
[0042] Example 2: Synthesis of 2-N,N-dimethylcarbamoyl-1-(4-methylphenyl)-3-phenyl-1,3-propanedione
[0043] Using 1-(4-methylphenyl)-3-phenyl-1,3-propanedione and N,N-dimethylformamide as raw materials, the reaction steps are as follows:
[0044] Add 1-(4-methylphenyl)-3-phenyl-1,3-propanedione (0.238 g, 1 mmol), N,N-dimethylformamide (2 ml), Copper chloride (0.013 g, 0.1 mmol) and tert-butyl hydroperoxide (0.180 g, 2 mmol) were reacted at 110°C;
[0045] TLC tracking reaction until complete completion;
[0046] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 87%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.03 – 7.94 (m, 1H), 7.92 – 7.88 (m, 1H), 7.77 – 7.69 (m, 1H), 7.66 – 7.55 (m, 1H), 7.55 – 7.35 (m, 3H), 7.33 – 7.20 (m , 2H), 7.16 – 7.10 (m, 1H), 3.17 – 3.08 (m, 3H),3.04 – 2.98 (m, 3H), 2.43 – 2.32 (m, 3H).
Embodiment 3
[0047] Example 3: Synthesis of 2-N,N-dimethylcarbamoyl-1-(2-methylphenyl)-3-phenyl-1,3-propanedione
[0048] Using 1-(2-methylphenyl)-3-phenyl-1,3-propanedione and N,N-dimethylformamide as raw materials, the reaction steps are as follows:
[0049] Add 1-(2-methylphenyl)-3-phenyl-1,3-propanedione (0.238 g, 1 mmol), N,N-dimethylformamide (2 ml), Copper bromide (0.011 g, 0.05 mmol) and tert-butyl hydroperoxide (0.180 g, 2 mmol) were reacted at 120°C;
[0050] TLC tracking reaction until complete completion;
[0051] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 77%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.05 – 7.92 (m, 1H), 7.77 – 7.63 (m, 1H), 7.59 – 7.22 (m, 7H), 6.83 – 6.56 (m, 1H), 3.21 – 2.80 (m, 6H), 2.60 – 2.51 (m , 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com