Method for synchronous complex breaking and heavy metal removal based on self-strengthening ozone
A heavy metal, complex breaking technology, applied in chemical instruments and methods, oxidized water/sewage treatment, water/sewage multi-stage treatment, etc. and TOC removal, easy operation and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A kind of self-strengthening ozone decomplexation and the method for synchronously removing heavy metals of the present embodiment, heavy metal A and complexing agent B are copper and EDTA respectively, prepare Cu-EDTA waste water with tap water, molar ratio is Cu 2+ :EDTA=1:2,Cu 2+ The concentration is 0.5mmol / L.
[0042] Step 1. Adjust the pH of Cu-EDTA wastewater to 4.5 and then add it to the ozone contact tank. The temperature in the tank is 25°C. Continuously feed ozone through the micropores at the bottom of the ozone contact tank. The dosage is 30mg / (L(aq) .min), micropore diameter ≤ 15μm, auxiliary hydraulic circulation stirring to ensure uniform reaction, reaction 40min, the reaction steps are:
[0043] o 3 +Cu(Ⅱ)-EDTA 2- →Cu(Ⅱ)-EDTAradical+O 3 · -
[0044] HO·+Cu(Ⅱ)-EDTA 2- →Cu(Ⅱ)-EDTAradical+OH-
[0045] Cu(Ⅱ)-EDTAradical→Cu(Ⅲ)-EDTA -
[0046] Cu(Ⅲ)-EDTA - →Cu(Ⅱ)-Yradical+products
[0047] Cu(Ⅱ)-Yradical→Cu(Ⅰ)-Yradical+products
[0048] Cu(Ⅰ)-Yra...
Embodiment 2
[0057] The self-strengthening ozone decomplexing and synchronously removing heavy metal method of the present embodiment, its specific steps are the same as embodiment 1, the difference is: the molar ratio is Cu 2+ :EDTA=1:1, adjust the pH to 3.1, react for 30 minutes, the dosage of ozone is 20mg / (L(aq)·min), and the temperature of step 1 and step 2 is 35°C.
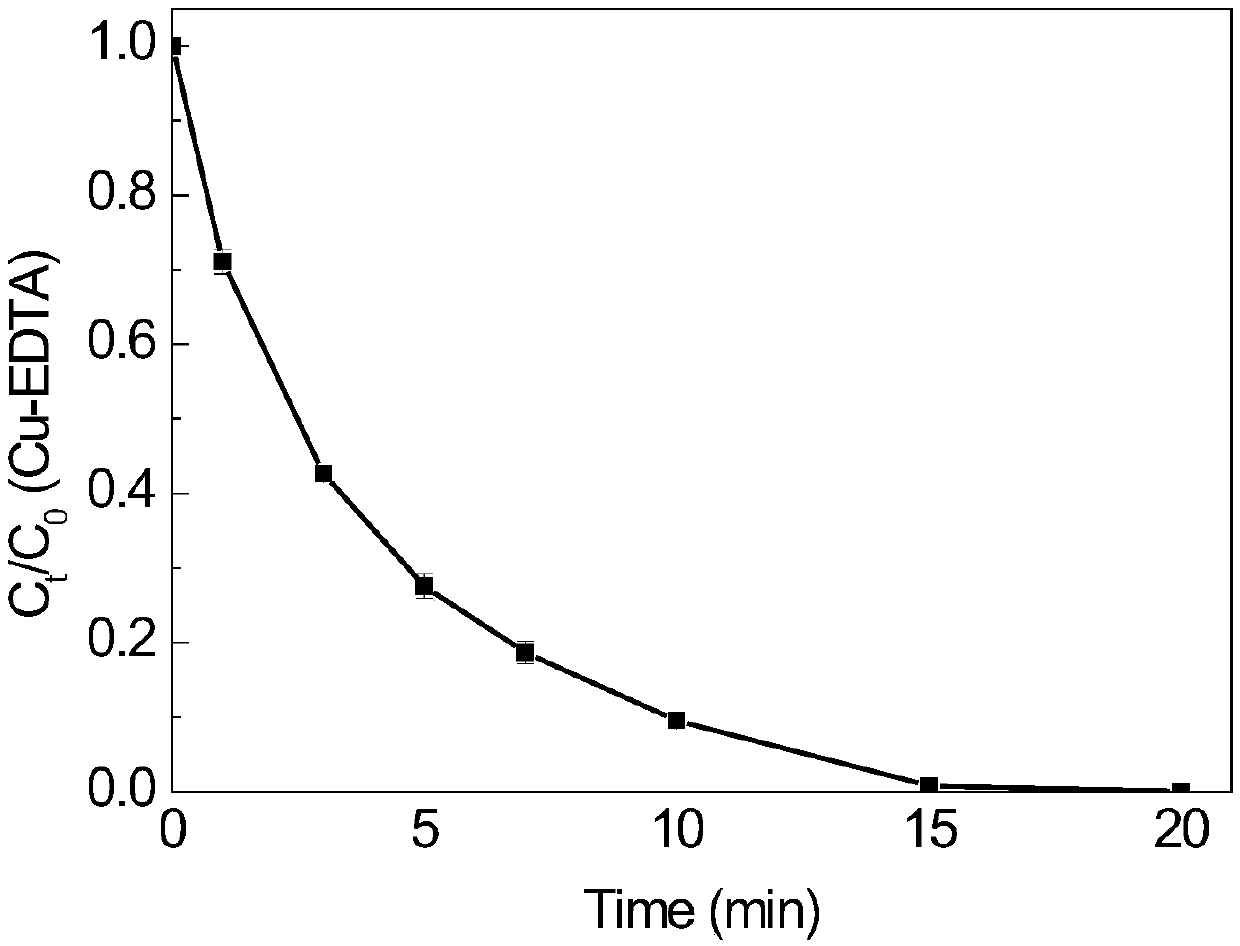
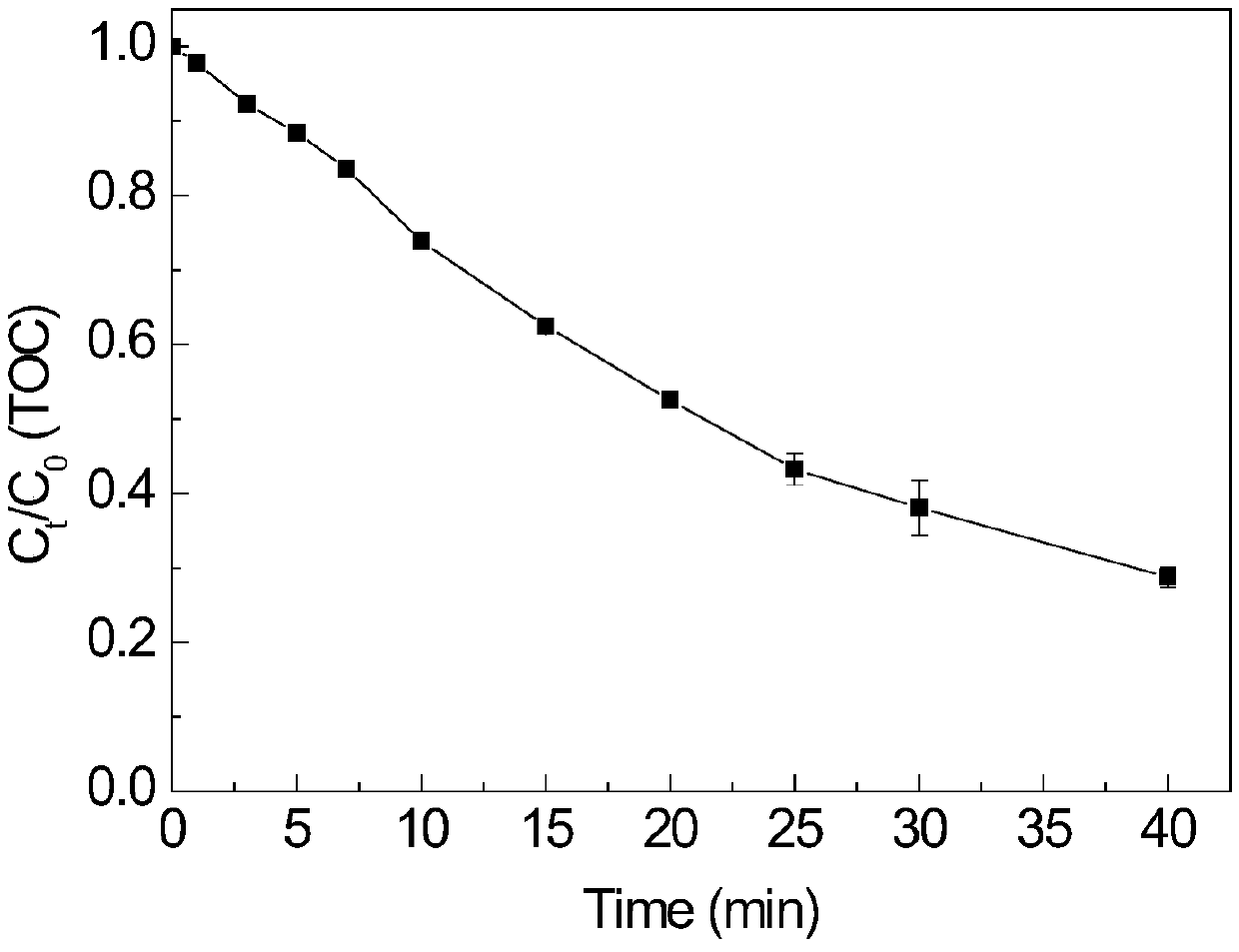
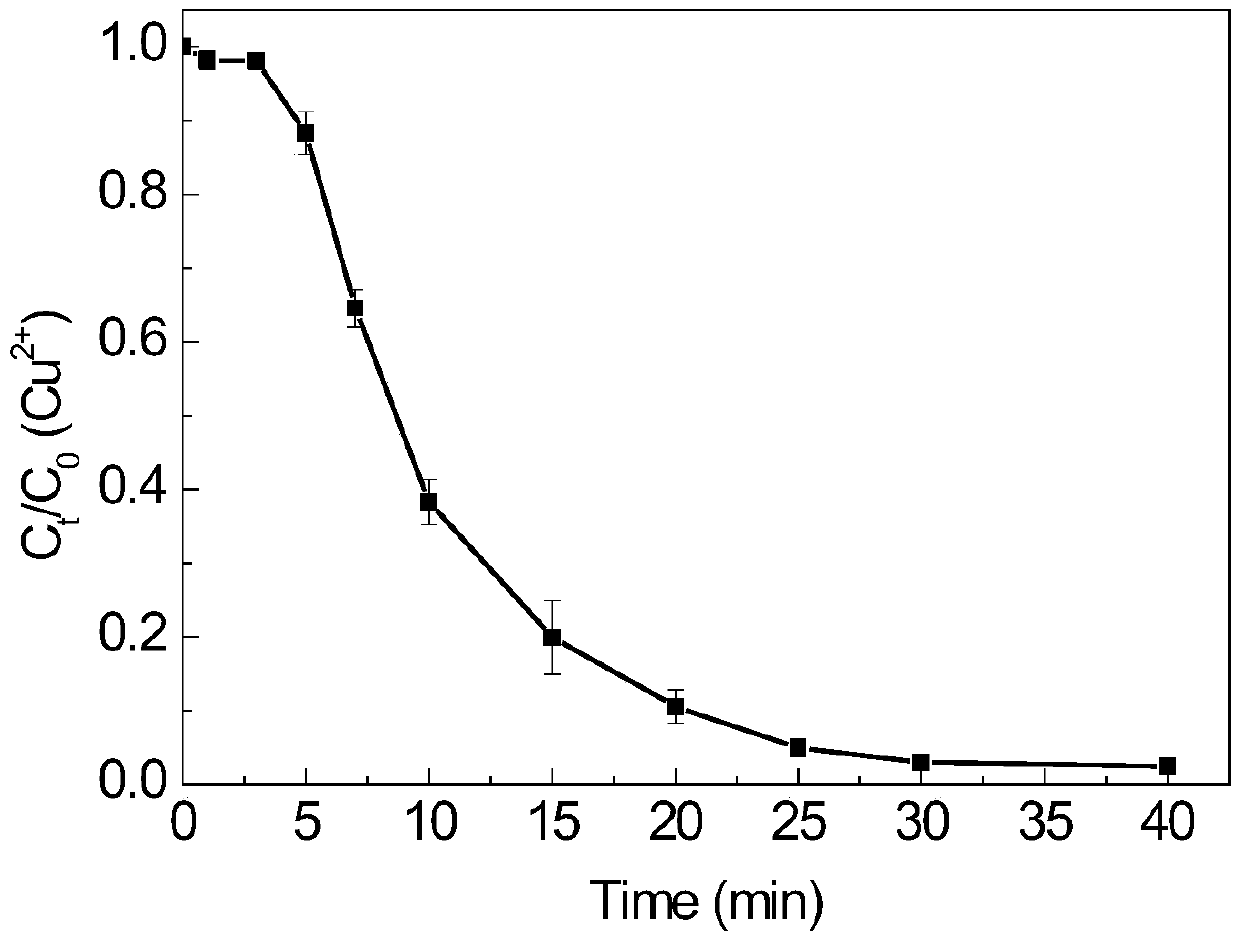
[0058] In this example, Cu-EDTA was completely degraded within 10 minutes, and after 30 minutes of reaction, the removal rate of TOC was about 70%, and the heavy metal Cu 2+ The removal rate is about 99%.
Embodiment 3
[0060] The self-strengthening ozone decomplexation and the method for synchronously removing heavy metals of the present embodiment, heavy metal A and complexing agent B are Ni and EDTA respectively, prepare Ni-EDTA waste water with tap water, molar ratio is Ni 2+ :EDTA=10:1,Ni 2+ The concentration is 0.75mmol / L.
[0061] The specific steps are the same as in Example 1, except that the pH is adjusted to 3.0; the reaction time is 10 minutes, the ozone dosage is 120 mg / (L(aq)·min), and the reaction temperature is 40°C.
[0062] In this example, Ni-EDTA was completely degraded within 5 minutes, and after 10 minutes of reaction, the removal rate of TOC was about 80%, and the heavy metal Ni 2+ The removal rate is about 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com