Dicarbazole derivative and organic electroluminescent element
A derivative, dicarbazole technology, applied in the field of novel dicarbazole derivatives, can solve the problems of insufficient current efficiency, insufficient current efficiency, low voltage driveability, etc., and achieve high luminous efficiency and power efficiency, and good hole injection. properties, wide bandgap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
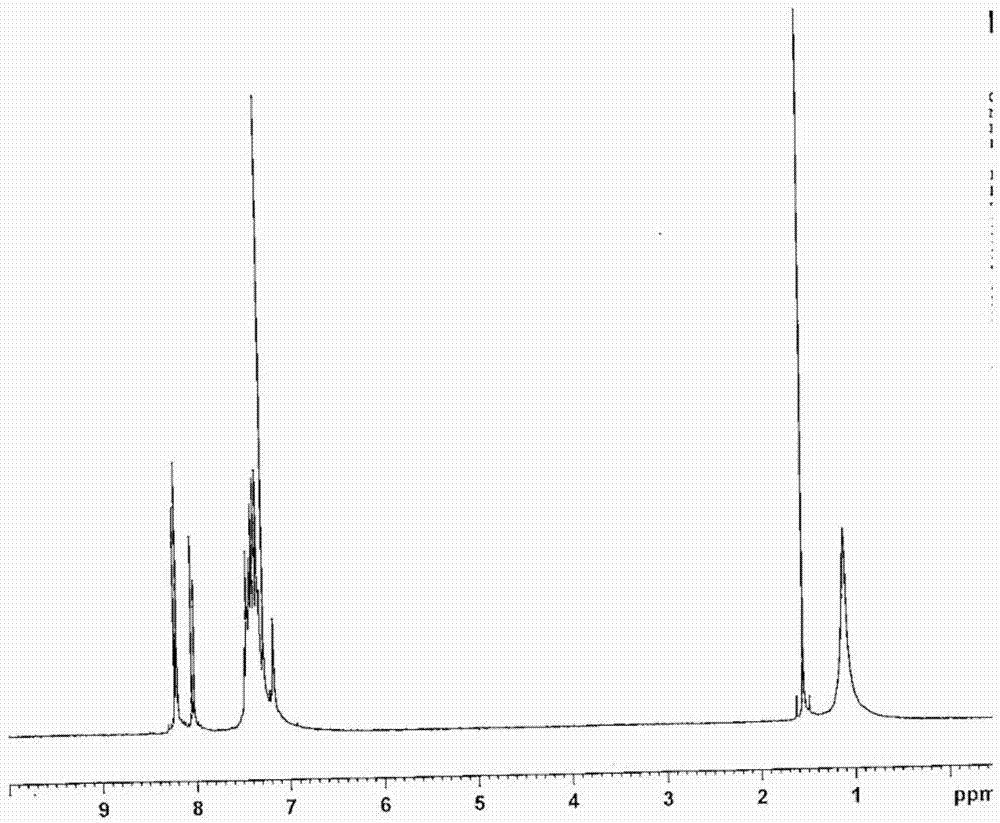
[0186] Synthesis of 5,7-dihydro-5,7-bis(9,9-dimethylfluoren-2-yl)-furo[2,3-a:5,4-a']dicarbazole
[0187] (Synthesis of compound 4)
[0188]
[0189] In a nitrogen-substituted reaction vessel,
[0190] With N,N,N',N'-tetramethylethylenediamine 10.4g, and
[0191] THF40ml
[0192] It was placed and cooled, and a hexane solution (1.6 mol / L) of n-butyllithium was added dropwise thereto while keeping the temperature of the solution at 0° C. or lower.
[0193] Further, the solution was stirred at 0°C for 30 minutes and at room temperature for another 30 minutes.
[0194] After that, 25 ml of THF and 5.0 g of dioxyfluorene were added thereto, and the mixture was heated and stirred at 60° C. for 2 hours. In the next step, 1,2-diiodoethane was added thereto while cooling to below -60°C, and thereafter, the mixture was stirred overnight at room temperature. After adding water and dichloromethane, the organic layer was extracted by a liquid separation operat...
Embodiment 2
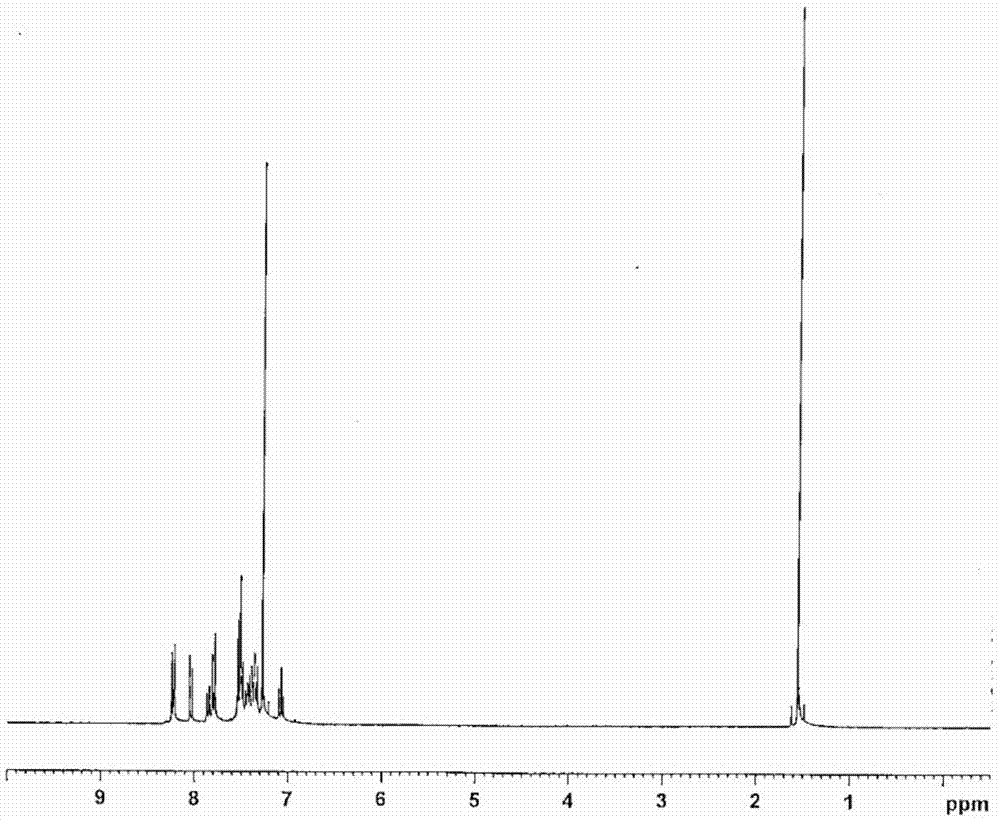
[0217] Synthesis of 5,7-dihydro-5,7-bis{4-(oxyfluoren-4-yl)phenyl}-furo[2,3-a:5,4-a']dicarbazole
[0218] (Synthesis of compound 5)
[0219]
[0220] To the nitrogen-purged reaction vessel, add:
[0221]
[0222] And the mixture was bubbled with nitrogen for 1 hour.
[0223] Afterwards, in the reaction vessel,
[0224] Add three (dibenzylidene acetone) dipalladium (O) 0.6g and
[0225] 0.8 g of a toluene solution containing 50% (w / v) of tri-tert-butylphosphine.
[0226] Then, the mixture was heated and stirred at 95°C for 18 hours. The mixture was cooled to room temperature, water was added thereto, extraction was performed using toluene, and an organic layer was extracted. The organic layer was dehydrated using anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain a crude product.
[0227] The crude product was purified by column chromatography (carrier: silica gel, eluent: toluene / n-hexane) to obtain 5,7-dihydro...
Embodiment 3
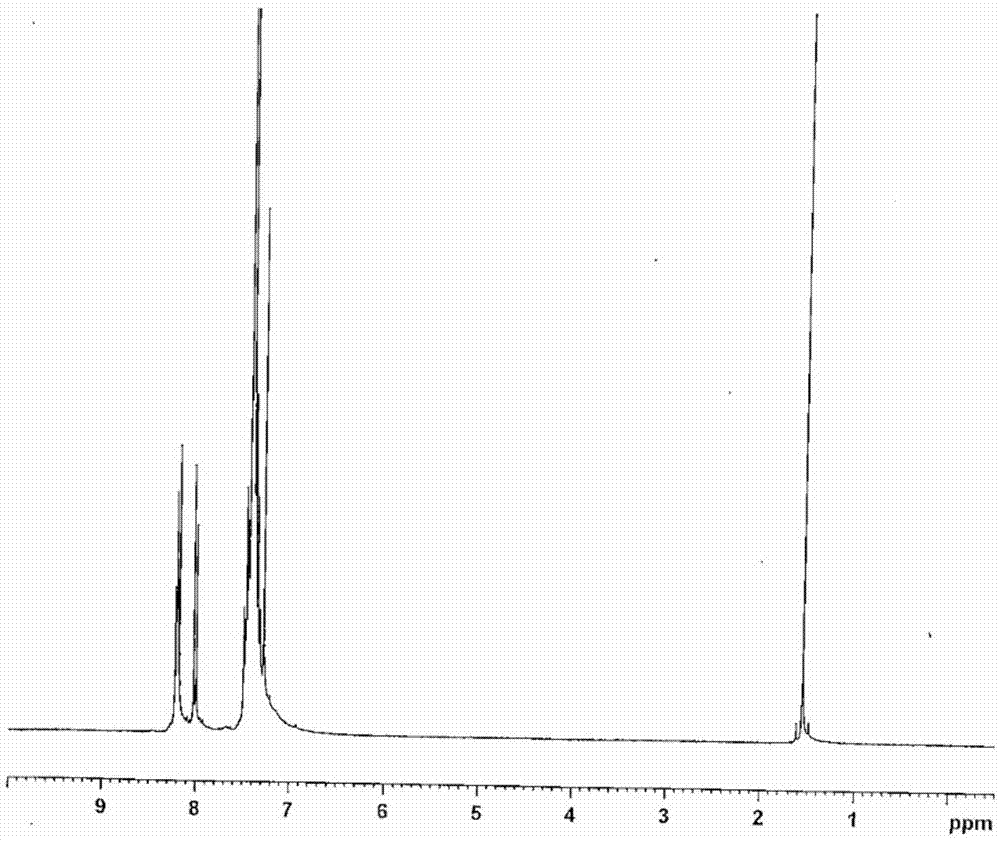
[0236] Synthesis of 5,7-dihydro-5,7-bis(biphenyl-4-yl)-furo[2,3-a:5,4-a']dicarbazole
[0237] (Synthesis of compound 6)
[0238]
[0239] To the nitrogen-purged reaction vessel, add:
[0240]
[0241] The mixture was bubbled with nitrogen for 1 hour.
[0242] Afterwards, in the reaction vessel,
[0243] Add tris(dibenzylideneacetone) dipalladium (0) 0.8g, and
[0244] 1.0 g of a toluene solution containing 50% (w / v) of tri-tert-butylphosphine.
[0245] Then, the mixture was heated and stirred at 95°C for 29 hours. The mixture was cooled to room temperature, water was added thereto, extraction was performed using toluene, and an organic layer was extracted. The organic layer was dehydrated using anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain a crude product.
[0246] The crude product is purified by column chromatography (carrier: silica gel, eluent: toluene / n-hexane) to obtain 5,7-dihydro-5,7-bis(biphenyl-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com