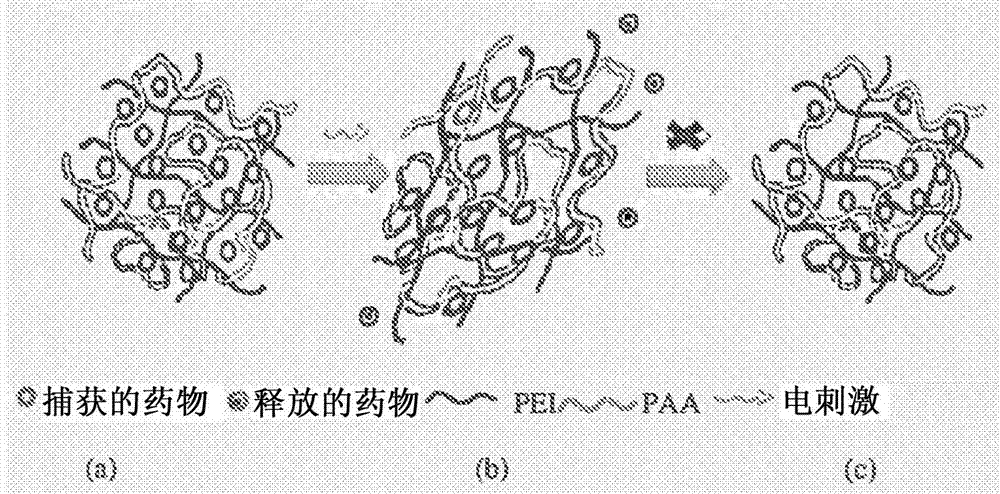
Rotating machine with at least one active magnetic bearing and spaced auxiliary rolling bearings
A technology of electrical stimulation and polymers, applied in drug combination, drug delivery, antipyretics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0169] 1. Fabrication and In Vitro Testing
[0170] Material
[0171] Polyethyleneimine (PEI) solution (M w 750,000), 1-vinylimidazole (1VA) (≥99%), indomethacin (≥99%), polyvinyl alcohol (PVA) (M w 89,000-98,000, 99+% hydrolyzed), acrylic acid (AA) (anhydrous, 99%), N,N'-methylenebisacrylamide (≥99.5%) and potassium persulfate (≥99.0%) are all from (St.Louis, USA) purchase. All other ingredients were of analytical grade and used as received.
[0172] Preparation of polymeric hydrogel pharmaceutical dosage forms
[0173] In order to manufacture the polymeric hydrogel pharmaceutical dosage form according to the invention, the following manufacturing method is used. A 6% polyvinyl alcohol (PVA)-1M sodium hydroxide solution was prepared, to which a polyethyleneimine (PEI) solution and 1-vinylimidazole (1VA) were added to form a mixture. Subsequently, the drug (100 mg - constant throughout all formulations and all instances of drug) was dissolved into the mixture. Acrylic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com