A primer, kit and detection method for detecting epidemic hematopoietic organ necrosis virus using pyrosequencing technology
A technology of hematopoietic organ necrosis and pyrosequencing, applied in the field of molecular biology, can solve problems such as false positives and inability to achieve rapid confirmation, and achieve the effects of small sample volume, accurate short DNA sequence analysis, and short time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 detects the design of the primers of epidemic hematopoietic organ necrosis virus
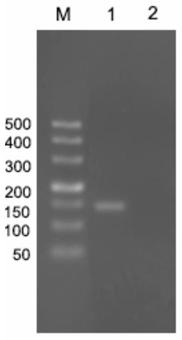
[0027] By comparing and analyzing the gene sequences of the epidemic hematopoietic organ necrosis virus published in the NCBI gene bank (GenBank) of the National Center for Bioinformatics in the United States, it was determined that the specific sequence of the EHNV ORF65 gene was 174bp, and specific PCR primers and primers were designed according to the specific sequence. Pyrosequencing primers, screen out a set of optimal primers for subsequent PCR and sequencing.
[0028] The epidemic hematopoietic organ necrosis virus specific primer designed according to the specific region sequence of the epidemic hematopoietic organ necrosis virus ORF65 gene of the present invention can amplify a specific single band for the epidemic hematopoietic organ necrosis virus DNA, and the PCR amplification fragment length It is 137bp. Then design pyrosequencing primers according to the specif...
Embodiment 2
[0033] Embodiment 2 kit composition
[0034] The kit includes the primer set designed in Example 1 for detecting the epidemic hematopoietic organ necrosis virus, that is, the amplification primer pair and the sequencing primer. The kit also includes the materials and reagents needed to complete the PCR reaction and pyrosequencing, such as DNA extraction reagents (DNeasy Blood & Tissue Kit, QIAGEN Company), PCR reaction reagents (PyroMark PCR Kit, QIAGEN Company), single-stranded template preparation reagents (Strand Mycoavidin-coated magnetic beads, GE Company), pyrosequencing reagents (PyroMark Gold Q96SQA Reagents, denaturing buffer, washing buffer, QIAGEN Company), etc., the above materials and reagents can be mixed and packaged, or individually packaged, Preferably, it is packaged individually.
Embodiment 3
[0035] The establishment of embodiment 3 detection method
[0036] 1. DNA extraction of epidemic hematopoietic organ necrosis virus
[0037] Using EHNV (the EHNV strain donated by the Danish Veterinary Laboratory of OIE Fish Disease Reference Laboratory) as raw material, DNeasyBlood&Tissue Kit (QIAGEN company, product number 69506) was used to extract DNA. The kit operation steps are as follows:
[0038] (1) Pipette 20 μl proteinase K to the bottom of a 1.5ml centrifuge tube. (2) Add 200 μl of the virus liquid to be extracted into the centrifuge tube. (3) Add 200 μl Buffer AL to the sample, and vortex for 15 seconds to mix. (4) Incubate at 56°C for 10 minutes. (5) Centrifuge quickly to remove the liquid droplets remaining in the lid of the 1.5ml centrifuge tube. (6) Add 200 μl (equal volume of the sample) of ethanol (96-100%), and vortex for 15 seconds to mix. After shaking, quickly centrifuge to remove the liquid droplets remaining in the cap of the 1.5ml centrifuge tube...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com