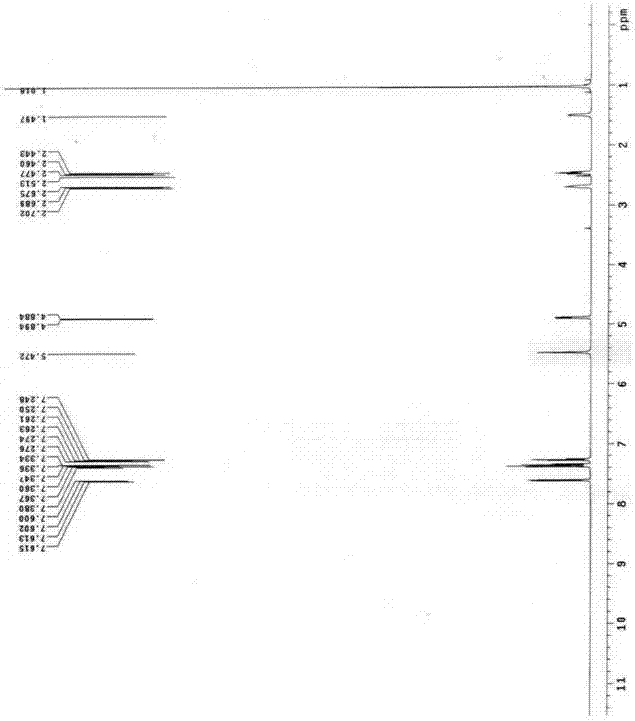
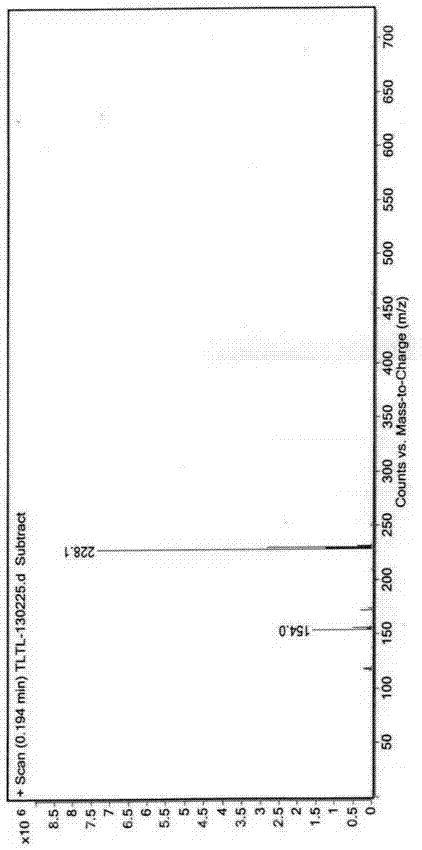
Synthesis method for compound tulobuterol
A technology of tulobuterol and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of hidden safety hazards and high cost, and achieve the effects of improving production efficiency, less side reactions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0050] The preparation of embodiment 12-chlorostyrene
[0051]78.3g (0.5mol) of 1-(2-chlorophenyl)-1-ethanol, 40.8g potassium bisulfate (0.3mol) and 5.5g hydroquinone (0.05mol) were added in a 500ml single-necked bottle, and then React at 190-195°C for 3 hours. After cooling down to room temperature, slowly pour the reaction system into 300ml of diethyl ether under stirring, spin the diethyl ether to dryness, add 200ml of dichloromethane and 100ml of water, stir well, separate the water layer, and wash the organic layer 3 times with saturated brine (50ml × 3), after drying, filtering and concentrating over anhydrous sodium sulfate, 61.2 g of 2-chlorostyrene was obtained as a colorless oil, with a yield of 88%.
Embodiment 22
[0052] The preparation of embodiment 22-chlorostyrene
[0053] 78.3g (0.5mol) of 1-(2-chlorophenyl)-1-ethanol, 54.5g potassium bisulfate (0.4mol) and 11.0g hydroquinone (0.1mol) were added in a 500ml single-necked bottle, and then React at 200-205°C for 3 hours. The aftertreatment method was the same as in Example 1 to obtain 62.6 g of the product, with a yield of 90%.
Embodiment 32
[0054] The preparation of embodiment 32-chlorostyrene
[0055] 78.3g (0.5mol) of 1-(2-chlorophenyl)-1-ethanol, 47.7g potassium bisulfate (0.35mol) and 8.3g hydroquinone (0.075mol) were added in a 500ml single-necked bottle, and then React at 210-215°C for 3 hours. The post-treatment method was the same as in Example 1 to obtain 58.4 g of the product, with a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com