Preparation method of 3,4,5-trifluorobromobenzene
A technology of trifluorobromobenzene and trifluoroaniline, which is applied in the preparation of pharmaceutical intermediates and the field of pesticides, can solve the problems of potential safety hazards, low reduction yield, and many impurities, and achieve the effect of reducing usage and solving high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of 3,4,5-trifluorobromobenzene
[0024] Put 500 milliliters of water in the reactor, 147 grams (1 mole) of 2,3,4-trifluoroaniline, 214.5 grams (mass concentration is 98%, 3.5 moles) of glacial acetic acid, control the temperature in the reactor at 25~30 ℃ 450 grams of sulfuric acid (mass concentration: 98%, 4.5 moles) was added dropwise, and after the addition was completed, the temperature in the reactor was controlled to react at 25-30° C. for 1 hour.
[0025] Control the temperature in the reactor to be 35-40°C and add 79.9 grams (0.5 moles) of bromine dropwise for about 4 hours, then control the temperature in the reactor to be 35-40°C and add hydrogen peroxide and water for dilution , The dropping time is controlled within 2-2.5 hours, and the reaction is kept warm after the dropping is completed. During the heat preservation reaction process, the product mixture obtained by the heat preservation reaction is sampled, the sample is neu...
Embodiment 2
[0030] Embodiment 2: the preparation of 3,4,5-trifluorobromobenzene
[0031] Example 1 was repeated except that no water for dilution was added when hydrogen peroxide was added dropwise to obtain 181.9 g of product 3,4,5-trifluorobromobenzene with a purity of 97.1% and a yield of 83.7%.
[0032] The inventor found unexpectedly that in the process of preparing 2,3,4-trifluoroaniline sulfate by reacting 2,3,4-trifluoroaniline, adding water for dilution while adding hydrogen peroxide dropwise, the product 3 , the yield of 4,5-trifluorobromobenzene has a certain influence.
Embodiment 3
[0033] Embodiment 3: the preparation of 3,4,5-trifluorobromobenzene
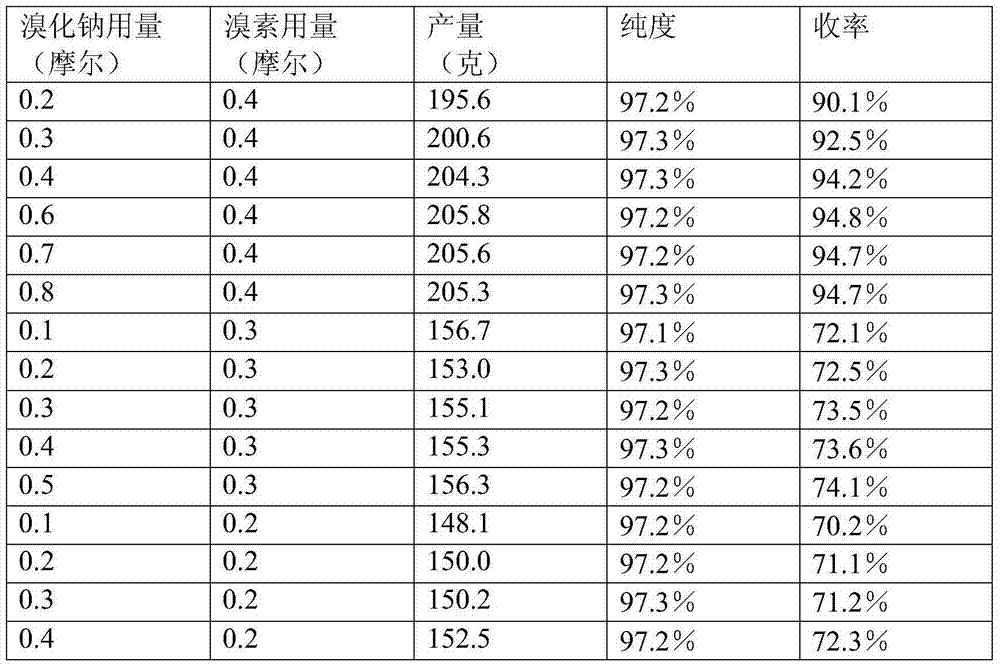
[0034] Repeat Example 1, the difference is that the operation of "dropping bromine 79.9 grams (0.5 moles)" is replaced by "dropping bromine 95.8 grams (0.6 moles)" to obtain the product 3,4,5-trifluorobromobenzene 205.6 grams, the purity is 97.2%, and the yield is 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com