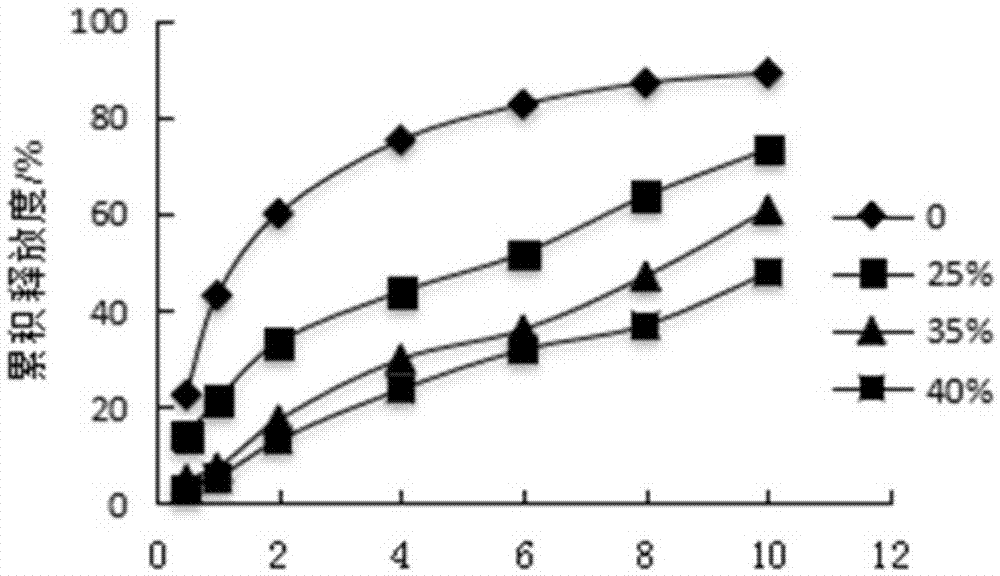
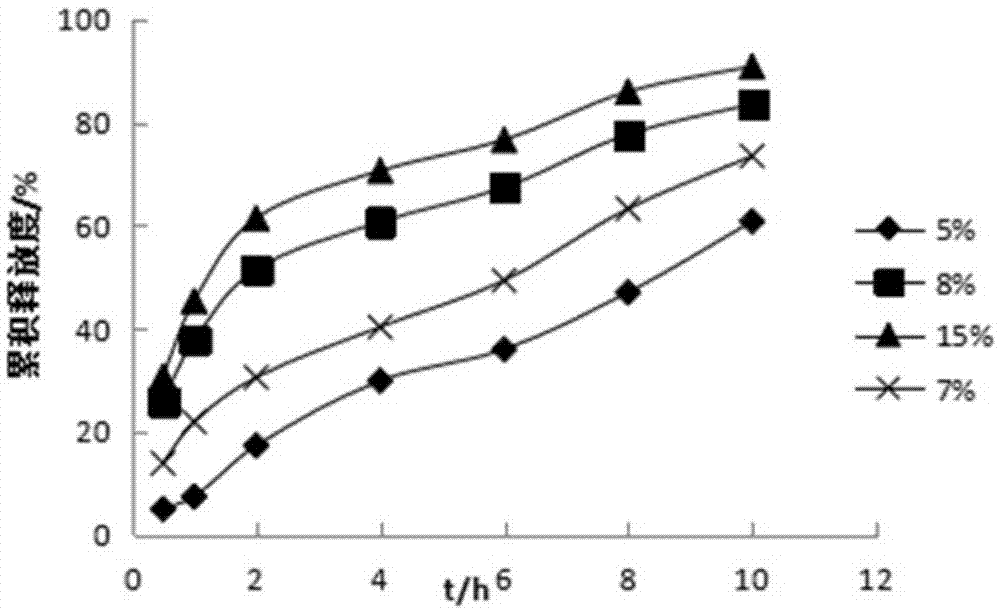
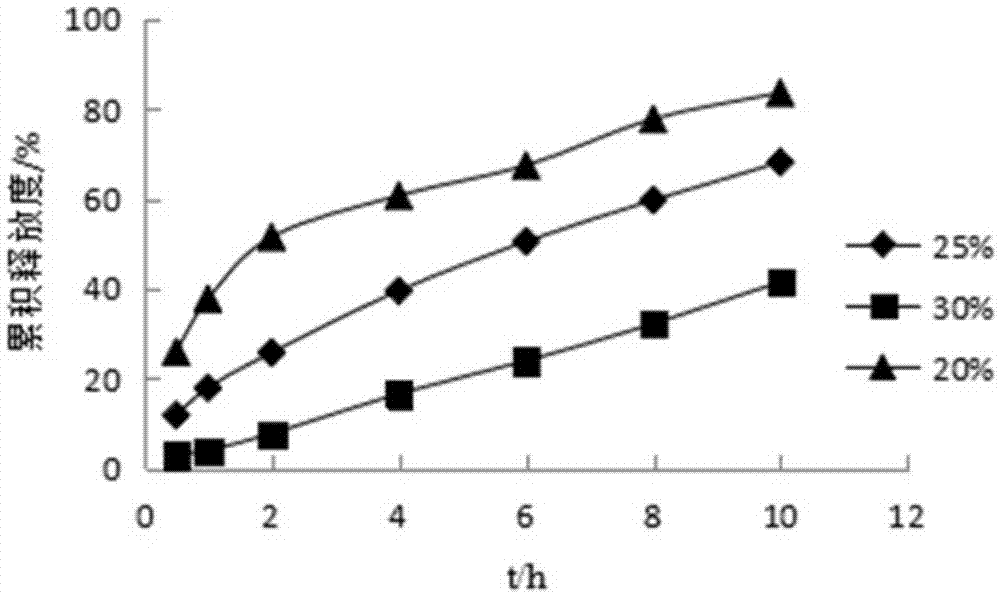
Pyridostigmine bromide coated sustained-release pellets and preparation method thereof
A technology of pyridostigmine and sustained-release pellets, applied in the field of pyridostigmine-coated sustained-release pellets and its preparation, can solve the problems of no pyridostigmine, unstable curative effect, and inconvenience for patients to take , to achieve good clinical application prospects and social benefits, reduce the frequency of medication, and the effect of ideal sustained release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 The preparation of pyridostigmine bromide-coated sustained-release pellets of the present invention
[0040] The prescription is as follows:
[0041] The weight ratio of raw and auxiliary materials in the plain pill is:
[0042]
[0043] The weight ratio of auxiliary materials in the sustained-release coating layer is:
[0044]
[0045] The preparation process is as follows:
[0046] a. Preparation of vegetarian pill coating solution: use ethanol as solvent, dissolve 20mp.s ethyl cellulose in 150ml of 95% ethanol, and ultrasonically swell and dissolve it; add purified water to dilute to 200ml; add bromine in the prescribed amount respectively Dissolve pyridostigmine, then add diethyl phthalate to dissolve, and prepare plain pill coating solution for use; mix the blank ball core with talcum powder, and pass through an 80-mesh sieve to remove excess talcum powder.
[0047] b. Preparation of bromipyrizine pills: Coating with fluidized bed, inlet air t...
Embodiment 2
[0050] Embodiment 2 The preparation of pyridostigmine bromide-coated sustained-release pellets of the present invention
[0051] The prescription is as follows:
[0052] The weight ratio of raw and auxiliary materials in the plain pill is:
[0053]
[0054] The weight ratio of auxiliary materials in the sustained-release coating layer is:
[0055]
[0056] The preparation process is as follows:
[0057] a. Preparation of vegetarian pill coating solution: use ethanol as solvent, dissolve 20mp.s ethyl cellulose in 150ml of 95% ethanol, and ultrasonically swell and dissolve it; add purified water to dilute to 200ml; add bromine in the prescribed amount respectively Dissolve pyridostigmine, then add diethyl phthalate to dissolve, and prepare plain pill coating solution for use; mix the blank ball core with talcum powder, and pass through an 80-mesh sieve to remove excess talcum powder.
[0058] b. Preparation of bromipyrizine pills: Coating with fluidized bed, inlet air tem...
Embodiment 3
[0061] Embodiment 3 Preparation of pyridostigmine bromide-coated sustained-release pellets of the present invention
[0062] The prescription is as follows:
[0063] The weight ratio of raw and auxiliary materials in the plain pill is:
[0064]
[0065] The weight ratio of auxiliary materials in the sustained-release coating layer is:
[0066]
[0067] The preparation process is as follows:
[0068] a. Preparation of vegetarian pill coating solution: use ethanol as solvent, dissolve 20mp.s ethyl cellulose in 150ml of 95% ethanol, and ultrasonically swell and dissolve it; add purified water to dilute to 200ml; add bromine in the prescribed amount respectively Dissolve pyridostigmine, then add diethyl phthalate to dissolve, and prepare plain pill coating solution for use; mix the blank ball core with talcum powder, and pass through an 80-mesh sieve to remove excess talcum powder.
[0069] b. Preparation of Mingsu pills of bromide: coat with a fluidized bed, the air inle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com