A kit for culturing huc-msc step by step and huc-msc obtained by using the kit
A kit and culture medium technology, applied in the field of serum-free step-by-step culture kits, can solve the problems of stem cell adhesion, maintenance of proliferation stability and other characteristics that cannot achieve the expected results, non-existence of transmission of heterogeneous pathogens, and complex non-human serum components To achieve good proliferation ability and multi-directional differentiation potential, eliminate the risk of spreading heterogeneous pathogens, and shorten the primary culture time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Culture of hUC-MSC in conventional medium with serum
[0059] Test medium: 89 parts by volume of α-MEM, 10% fetal bovine serum (FBS), 100 U / ml penicillin, 100 U / ml streptomycin, 0.1 parts by volume of β-mercaptoethanol, 10 ng / ml of b-FGF , 1 part by volume of non-essential amino acid aqueous solution (11140, Gibco Company).
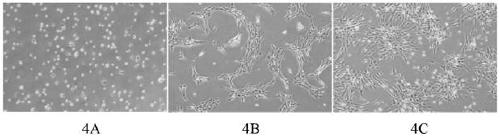
[0060] In the biosafety cabinet, the hUC-MSCs of the third generation isolated from the umbilical cord Huatong glue tissue of natural delivery newborns were collected and divided into 2×10 4 cells / cm 2 Density inoculation in T75 cell culture flask, add 15mL test medium, transfer into CO 2 The concentration is 5% in a 37°C constant temperature incubator. Observe the cell adhesion 2 hours after inoculation, a large number of hUC-MSC cells adhere to the wall, and tentacles protrude; observe after 48 hours, hUC-MSC reaches 90% confluence; the cell tentacles are stretched and bright.
[0061] For cell morphology see figure 1 . However...
Embodiment 2
[0062] Example 2 Culture hUC-MSC in conventional commercially available serum-free medium
[0063] Referring to the method in Example 1, inoculate with the same cell source and the same density, and add 15 mL of commercially available serum-free medium (product of Saiye Company, product number HUXUC-90061) to culture the cells. 2 hours after inoculation, it was observed that the cells were attached to the wall, the cells were bright, mostly round, and the antennae were stretched; 24 hours after the inoculation, the cells were observed, hUC-MSCs were bright under the microscope, the antennae were extended, and the expansion was not obvious; after 48 hours of inoculation Finally, the confluence rate of the cells reached about 50%. Observing the cells 72 hours after inoculation, the hUC-MSC cells were bright and reached more than 90% confluence. The cells were collected by trypsinization and frozen.
[0064] Optionally, after the cells reach 100% confluency, the cells begin to...
Embodiment 3
[0065] Example 3 Screening of medium composition
[0066] (1) Screening of TME medium composition
[0067] Test medium: 0.01, 0.02, 0.05, 0.1, 0.15, 0.2, 0.3 or 0.5 parts by volume of β-mercaptoethanol, 1 part by volume of non-essential amino acid aqueous solution (11140, Gibco), 99 parts by volume of a-MEM .
[0068] Referring to the method in Example 1, inoculate with the same cell source and the same density, and add 15 mL of test medium to culture the cells. Observe the cell adhesion.
[0069] Results: In the two concentration groups containing 0.01 and 0.02 parts by volume of β-mercaptoethanol in the medium, the speed of cell attachment was slow. After 4 hours of inoculation, some cells still did not adhere to the wall. After about 8 hours, The cells basically adhered to the wall; in the four concentration groups containing 0.05, 0.1, 0.15 and 0.2 volume parts of β-mercaptoethanol in the medium, the cells were completely adhered to the wall after 4 hours of inoculati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com