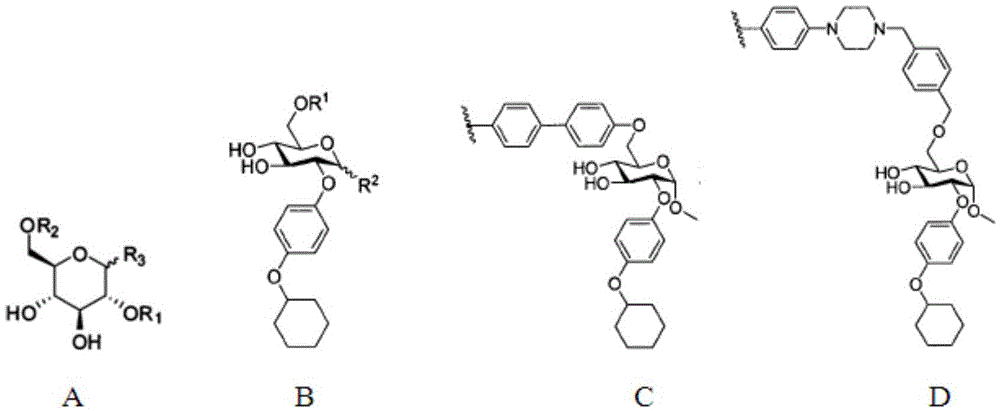
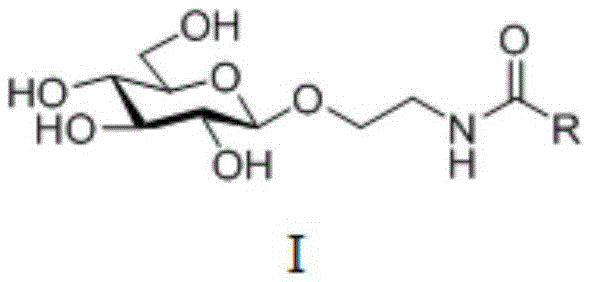
Amide ethyoxyl-beta-D-glucoside compound and preparation method and application thereof
A technology of glucoside and ethoxy, which is applied in the field of pharmaceutical compounds to achieve the effect of simple steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
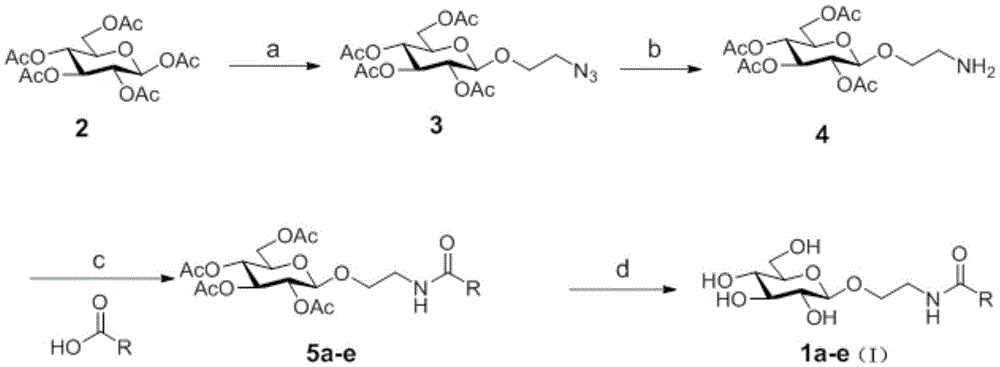
[0033] Embodiment 1 The preparation of preferred target compound 1a-e of the present invention
[0034] (1) Synthesis of 1-azidoethoxy-2,3,4,6-tetra-O-acetyl-β-D-glucoside (Compound 3)
[0035] Compound 2 (5g, 12.8mmol), azide ethanol (2.22g, 25.6mmol), molecular sieves (5g) and dichloromethane (30ml) were placed in a 100ml eggplant-shaped flask, and after stirring for 1 hour under argon protection, Cool to 0°C, slowly add boron trifluoride diethyl ether (6.47ml, 51.2mmol) dropwise, and stir at room temperature for 2 hours. TLC (petroleum ether: ethyl acetate=2:1, R f =0.45) detection, the reaction is complete. Dichloromethane (200 ml) was added to the reaction solution, and the mixture was washed with saturated sodium bicarbonate solution and saturated sodium chloride solution, respectively. The organic layer was dried over anhydrous sodium sulfate and concentrated. Purification by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) gave a white solid ...
Embodiment 2
[0046] Embodiment 2 Pharmacological experiments of preferred target compounds 1a-e of the present invention
[0047] (1) Experimental materials
[0048] 1. Test sample
[0049] After the target compounds were dissolved in DMSO (Merck), PBS (–) was added to make a 1000 μg / ml solution or a homogeneous suspension, and then diluted with DMSO-containing PBS (–).
[0050] 2. Cell lines
[0051] Human non-small cell lung cancer cells (H460), human liver cancer cells (HEP3B).
[0052] 3. Other materials and main instruments
[0053] Culture medium: RPMI1640 + 15% NBS + double antibody; thiazolium blue (MTT): purchased from Sigma Company (St.Louis, MO, USA); other reagents were domestic analytical grade. Fully automatic microplate reader WellscanMK-2 (Labsystems company), CO 2 Constant temperature incubator (Japan Sanyo Company), imported 96-well culture plate, etc.
[0054] (2) Experimental method
[0055] Both of the above two cell lines were cultured in DMEM medium containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com