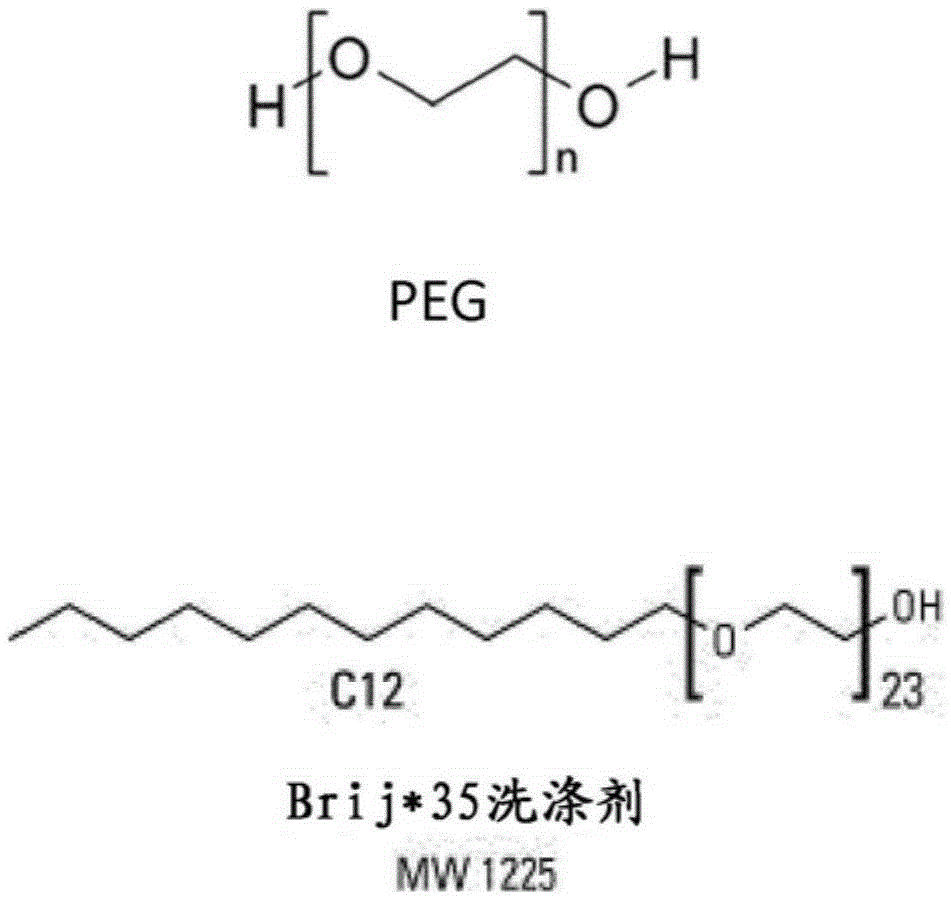
Milk clotting aspartic protease enzyme composition
A technology of aspartic acid and protease, applied in the direction of enzyme, peptidase, hydrolase, etc., can solve the problem that milk coagulase is not clearly mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0269] Embodiment 1: the mensuration of curd specific activity
[0270] 4.1 Determination of coagulation activity
[0271] Milk-clotting activity was determined using the REMCAT method, a standard method developed by the International Dairy Federation (IDF method).
[0272] Coagulation activity is determined by the time required for visible flocculation to occur on a standard milk substrate prepared from reduced heat, low fat milk powder using 0.5 g per liter of sodium chloride solution (pH ≈ 6.5). The clotting times of the milk coagulating enzyme samples were compared to a reference standard with known milk clotting activity and based on IDF standard 110B having the same enzyme composition as the samples. Samples and reference standards are measured under the same chemical and physical conditions. Variant samples were adjusted to approximately 3 IMCU / ml using 84 mM acetic acid pH 5.5 buffer. Thereafter, 200 μl of enzyme were added to 10 ml of pre-heated milk (32°C) place...
Embodiment 2
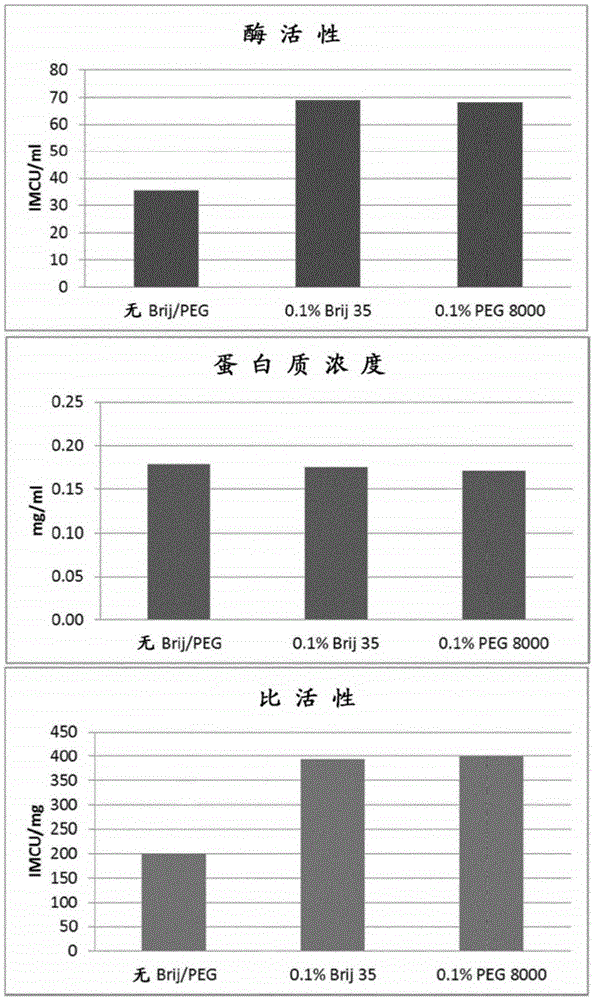
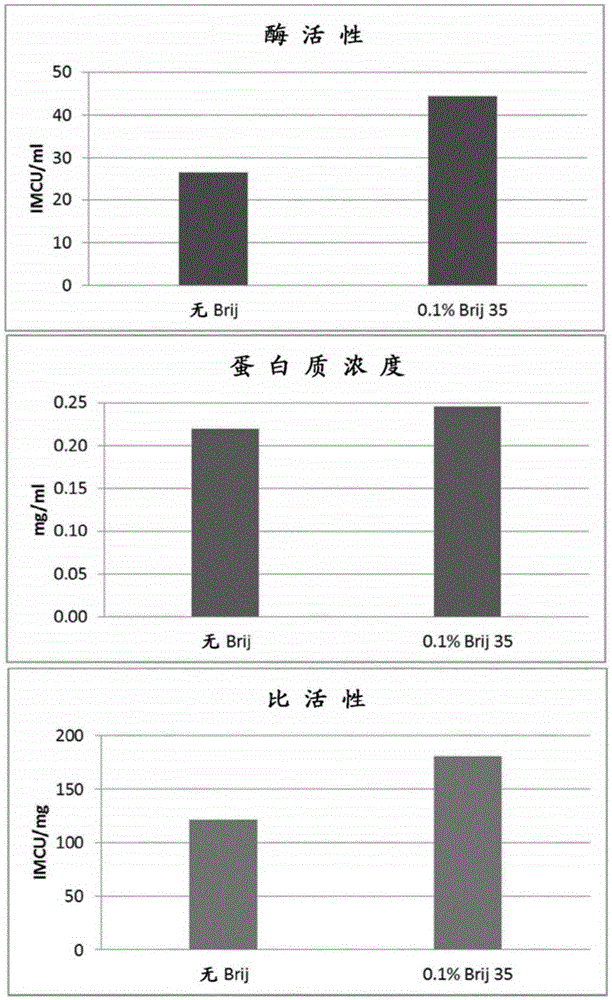
[0284] Example 2: Add PEG or Brij-35 to the elution buffer
[0285] Bovine chymosin or camelid chymosin was expressed recombinantly in Aspergillus niger (approximately as described in WO02 / 36752A2).
[0286] The enzyme was purified by a solid phase extraction method using a benzylamine ligand covalently bound to agarose (similar to that described in WO 01 / 58924A2).
[0287] 96-well filter plates equipped with 25 μm PE filters and 2 ml pore volume were packed with Fastline 1300 from Upfront Chromatography, Denmark.
[0288] The wells were filled with resin to obtain a bed height of 6-8 mm in all wells. The resin in all wells was equilibrated with 5 ml of 20 mM sodium malonate pH 5.7.
[0289] The supernatant from the culture was adjusted by mixing 3ml supernatant with 0.5ml 2M sodium malonate pH 5.7. 3.5 ml samples were then filtered through an 8 μm filter to remove particles and loaded into 96 individual wells of the plate. After loading, the resin was washed with 5 ml o...
Embodiment 3
[0304] Example 3: Addition of PEG to formulation (after purification)
[0305] A liquid clotting aspartic protease composition is obtained comprising clotting aspartic protease at a strength of about 1100 IMCU / g.
[0306] Compositions (ie 3 different compositions) were obtained for bovine chymosin, camelid chymosin and mucor pepsin.
[0307] PEG8000 was added to each composition to reach a level of 0.015% w / w (150 ppm w / w).
[0308] The control composition is the composition without adding PEG8000.
[0309] The compositions were stored at 5°C and 37°C for 6 months and periodically analyzed for curdling activity.
[0310] The results confirmed that after 6 months storage of the tested liquid milk-clotting aspartic protease composition , Addition of PEG increases long-term storage stability—— That is, these compositions have higher IMCU / g activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com