Peptide identification method by using mesoporous silica composite combined with mass spectrum
A technology of mesoporous silica and composite materials, applied in chemical instruments and methods, alkali metal compounds, alkali metal oxides/hydroxides, etc., to achieve the effects of large specific surface area, strong affinity, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
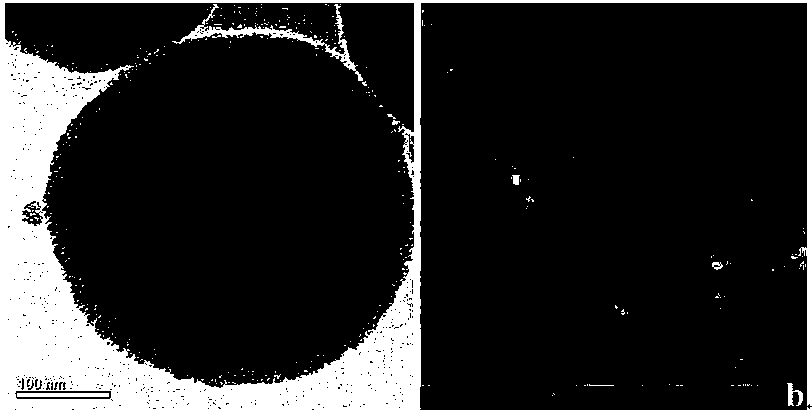
[0032] Example 1: Synthesis of a perfluoroalkyl-modified mesoporous silica composite material coated on the surface of a magnetic microsphere ferric oxide
[0033] (1) 1.35g ferric chloride hexahydrate (FeCl 3 ·6H 2 O) Dissolve in 75mL of ethylene glycol, stir magnetically until clarified, add 3.6g of sodium acetate, fully stir until dissolved, continue stirring for 0.5h, transfer to a hydrothermal synthesis reaction kettle, ultrasonicate for 5 minutes, then put in an oven Heat at 200°C for 16 hours, take out the reaction kettle, and cool for 10 hours; pour out the magnetic balls obtained from the reaction from the reaction kettle, wash them thoroughly with deionized water and absolute ethanol, and dry them under vacuum at 50°C;
[0034] (2) Disperse 75 mg of magnetic balls obtained in step (1) and 750 mg of cetyltrimethylammonium bromide in 75 mL of deionized water, and ultrasonicate for 30 minutes; -3 M sodium hydroxide solution was mixed, and a uniform and stable dispersi...
Embodiment 2
[0040] Example 2: The surface of the magnetic microsphere ferric oxide obtained in Example 1 is coated with a perfluoroalkyl-modified mesoporous silica composite material as a solid-phase microextraction adsorbent for the low concentration of perfluoroalkyl derivatization Enrichment and MALDI-TOFMS detection of β-casein hydrolyzate.
[0041] (1) Preparation of standard protein enzymatic hydrolysis solution: Accurately weigh 5mg of standard protein β-casein and dissolve in 25mM ammonium bicarbonate buffer, boil for 10 minutes, dilute to 1mg / mL with 25mM ammonium bicarbonate buffer, and then mix with protein Add an appropriate amount of trypsin at a volume ratio of 1:40, and enzymatically hydrolyze at 37°C for 16 hours.
[0042] (2) Derivation of standard proteolysis solution: Add 10 μL of dimethyl sulfoxide / ethanol solution (volume ratio 3:1) and 9.2 μL of saturated barium hydroxide solution to 10 μL of β-casein solution obtained in step (1) , 2 μL of sodium hydroxide solution...
Embodiment 3
[0048] Example 3: Synthesis of a perfluoroalkyl-modified mesoporous silica composite material coated on the surface of a magnetic microsphere ferric oxide
[0049] (1) 1.35g ferric chloride hexahydrate (FeCl 3 ·6H 2 O) Dissolve in 70mL of ethylene glycol, stir magnetically until clarified, add 5.0g of sodium acetate, stir fully until dissolved, continue stirring for 0.5h, transfer to a hydrothermal synthesis reaction kettle, ultrasonicate for 5 minutes, then put in an oven Heat at 190°C for 18 hours, take out the reaction kettle, and cool for 12 hours; pour out the magnetic balls obtained from the reaction from the reaction kettle, wash them thoroughly with deionized water and absolute ethanol, and dry them under vacuum at 60°C;
[0050] (2) Disperse 50 mg of magnetic balls obtained in step (1) and 500 mg of cetyltrimethylammonium bromide in 50 mL of deionized water, and ultrasonicate for 30 minutes; -3 M sodium hydroxide solution was mixed, and a uniform and stable dispersi...
PUM
| Property | Measurement | Unit |
|---|---|---|
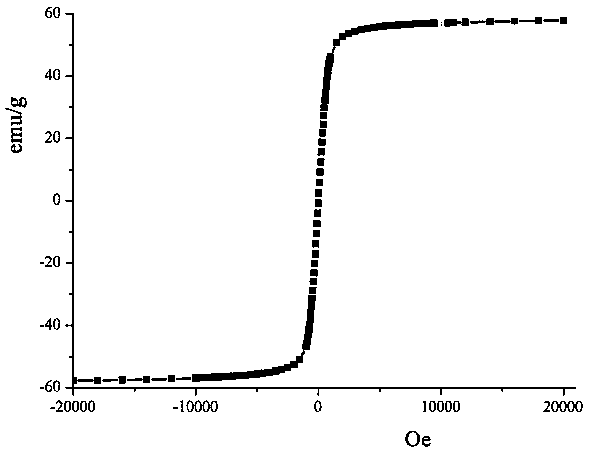
| Saturation magnetization | aaaaa | aaaaa |
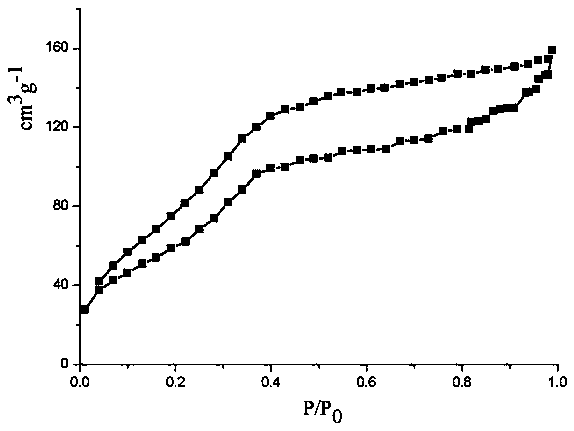
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com