R-2,2-di(2-thienyl)-2-glycolic acid quinine-3-ester and preparation and application thereof
A technology of quinine glycolate and methyl glycolate, which is applied in the field of R-2,2-di-2-hydroxyacetate quinine-3-yl ester, can solve the problem of high production cost, long reaction route and low yield and other problems, to achieve the effect of low cost, short reaction route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 3-quinine alcohol (38.1g, 0.3mol) and 600mL anhydrous toluene into a 1000mL three-necked flask, add 60% sodium hydride (13.2g, 0.33mol) in batches, stir at room temperature for 15min, then add 2-hydroxyl -2,2-bis(2-thienyl)acetic acid methyl ester (76.2g, 0.3mol), reflux reaction for 6h. After the reaction is complete, add 250 mL of water and stir for 20 min, separate the toluene layer, dry over anhydrous sodium sulfate and spin off the solvent to obtain the crude product. Ethanol was recrystallized to obtain 54.5 g of white solid, yield: 52.1%. Mp: 175~177℃. IR(KBr):3435,3075,2946,2876,2735,1732,1637,1566,1423,1354,1320,1232,1132,1085,997,837,784,689. 1 H-NMR (400MHz, DMSO-d 6 )δ:1.24(m,2H),1.54-1.58(m,3H),1.91(m,1H),2.45(m,1H),2.58-2.60(m,3H),3.07-3.36(m,1H) ,4.80-4.82(m,1H),6.69-7.02(m,2H),7.11-7.12(m,2H),7.36(m,1H),7.49-7.51(m,2H).
Embodiment 2
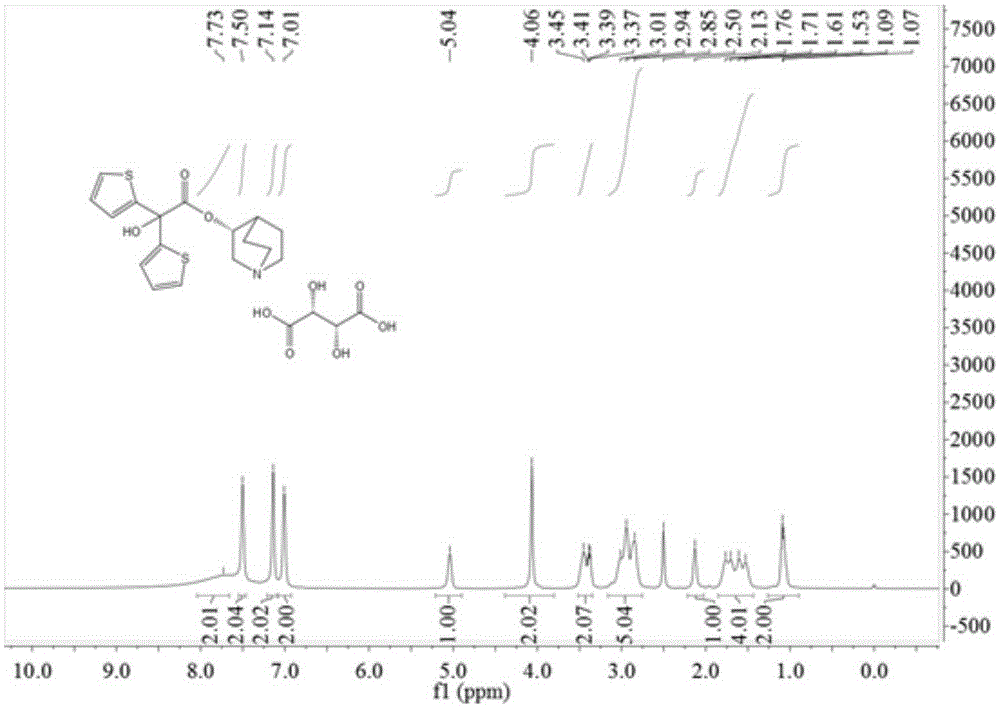
[0039] Add 2,2-bis(2-thienyl)-2-hydroxy-acetic acid-3-quinine ester (15.0g, 0.043mol), L-tartaric acid (6.45g, 0.043mol), 180mL of absolute ethanol was heated to reflux for 30min, and the reaction solution was clarified. After cooling to room temperature, a white solid precipitated out. Suction filtration yielded 8.4 g of white solid, with a yield of 39.1%. [α] D 25 =+116.1°(c=0.8g / 100mL, H 2 O). 1 HNMR (400MHz, DMSO-d 6 )δ: 1.08(d, J=6.4Hz, 2H), 1.65(dd, J=66.5, 27.4Hz, 4H), 2.13(s, 1H), 2.93(t, J=33.3Hz, 5H), 3.41( dd,J=19.3,12.0Hz,2H),4.06(s,2H),5.04(s,1H),7.01(s,2H),7.14(s,2H),7.50(s,2H),7.73(s ,2H).
[0040] Add 2,2-bis(2-thienyl)-2-hydroxy-acetic acid-3-quinine ester-L-tartrate prepared in the previous step to a 100mL eggplant-shaped bottle, 50mL of ethanol, and add sodium hydroxide solution to adjust the pH to 7-8, stirred at room temperature for 30 minutes, cooled, and extracted with chloroform. The solvent was removed to obtain 5.1 g of white solid R-2-hydro...
Embodiment 3
[0042] Add 3-quinine alcohol (25.4g, 0.2mol) and 500mL of anhydrous toluene into a 1000mL three-neck flask, add sodium methoxide (11.9g, 0.22mol) in batches, stir at room temperature for 15min, then add 2,2-dimethoxide in batches (2-Thienyl)methyl 2-hydroxyacetate (53.5g, 0.21mol) was refluxed for 8h. After the reaction was completed, add 250 mL of water and stir for 20 min, separate the toluene layer, dry over anhydrous sodium sulfate, evaporate the solvent to obtain a crude product. Recrystallization from ethanol gave 35.1 g of white solid 2,2-bis(2-thienyl)-2-hydroxy-acetic acid-3-quinine ester, yield 48.1%, mp: 176-178°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com