Synthetic method for 2-acetyl thiazole
A technology of acetyl thiazole and synthesis method, applied in directions such as organic chemistry, can solve problems such as high consumption of raw materials, uneconomical economy, complicated operation process, etc., and achieve the effects of improving preparation process, optimizing reaction temperature, and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
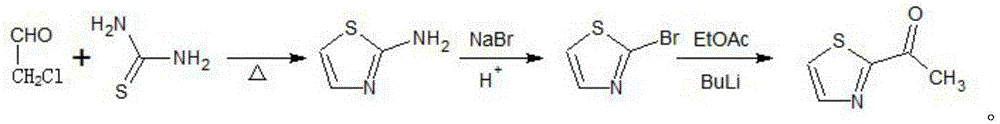
[0046] 1) Preparation of 2-aminothiazole
[0047] Add 300ml of toluene, 30g of thiourea and 40g of chloroacetaldehyde into a 500ml three-necked round-bottomed flask, stir, maintain the temperature at 60°C, keep the temperature for 1.5h, cool, add 20% sodium hydroxide solution for neutralization, adjust the pH to 7, separate The organic layer was distilled under reduced pressure to obtain 2-aminothiazole. The melting point of 2-aminothiazole was 92-93° C., the mass was 27.6 g, and the yield was 69.9%.
[0048] 2) Preparation of 2-bromothiazole
[0049] Dissolve 0.25mol of 2-aminothiazole in 150ml of 45% sulfuric acid in a 500ml reaction flask, stir to partially dissolve the 2-aminothiazole and cool to 2°C, add 50ml of 65% concentrated nitric acid dropwise, and keep the internal temperature below 10°C. Then slowly add 50ml of 5mol / l sodium nitrite aqueous solution dropwise, after the addition is complete, continue to stir for 1h, and control the temperature below 7°C. Use a dr...
Embodiment 2
[0053] 1) Preparation of 2-aminothiazole
[0054] Add 300ml of toluene, 30g of thiourea and 43g of chloroacetaldehyde into a 500ml three-neck round bottom flask, under stirring, maintain the temperature at 70°C, keep the temperature for 2.5h, cool, add 20% sodium hydroxide solution for neutralization, adjust the pH to 7, Separate the organic layer and distill under reduced pressure to obtain 2-aminothiazole, the melting point of 2-aminothiazole is 92-93°C, the mass is 31.5g, and the yield is 79.8%.
[0055] 2) Preparation of 2-bromothiazole
[0056] Dissolve 0.25mol of 2-aminothiazole in 150ml of 45% sulfuric acid in a 500ml reaction flask, stir to partially dissolve the 2-aminothiazole and cool to 4°C, add 50ml of 65% concentrated nitric acid dropwise, and keep the internal temperature below 10°C. After that, 50 mL of 5 mol / L sodium nitrite aqueous solution was slowly added dropwise. After the addition was completed, the stirring was continued for 1 h, and the temperature wa...
Embodiment 3
[0060] 1) Preparation of 2-aminothiazole
[0061] Add 300ml of toluene, 30g of thiourea and 46g of chloroacetaldehyde to a 500ml three-necked round bottom flask, under stirring, maintain the temperature at 80°C, keep the temperature for 2h, cool, add 20% sodium hydroxide solution for neutralization, adjust the pH to 7, divide The organic layer was taken out, and 2-aminothiazole was obtained by distillation under reduced pressure. The melting point of 2-aminothiazole was 92-93° C., the mass was 33.9 g, and the yield was 85.9%.
[0062] 2) Preparation of 2-bromothiazole
[0063] Dissolve 0.25mol of 2-aminothiazole in 150ml of 45% sulfuric acid in a 500ml reaction flask, stir to dissolve the 2-aminothiazole and cool to 5°C, add 50ml of 65% concentrated nitric acid dropwise, and keep the internal temperature below 10°C. Then slowly add 50ml of 5mol / l sodium nitrite aqueous solution dropwise, after the addition is complete, continue to stir for 1h, and control the temperature belo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com