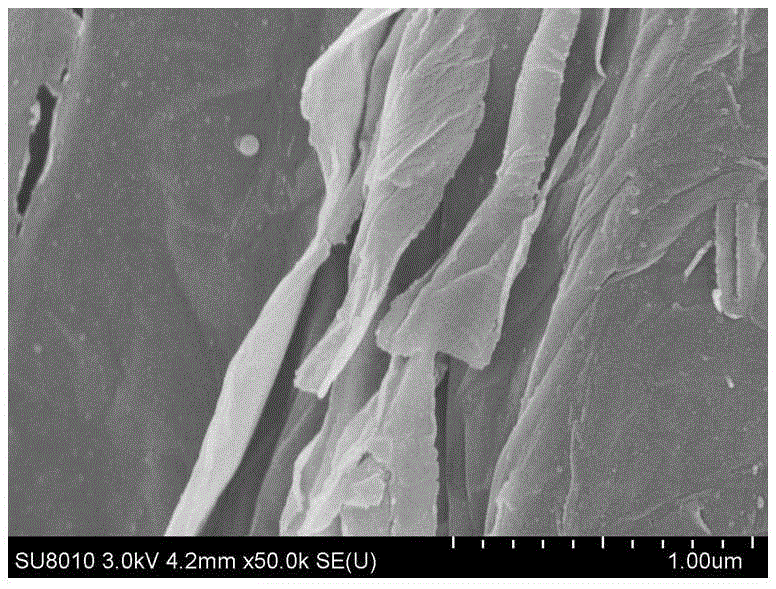
Preparation method of graphene oxide
A technology of graphene and rock oxide, which is applied in the field of preparing graphene oxide, to achieve the effects of reducing production costs, reducing waste acid and waste liquid, and controlling the degree of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] This embodiment illustrates that the graphite is 325 meshes of natural flake graphite, the alkali is KOH, and the pro-oxidant is KNO 3 reaction process and results.
[0018] Take 3g325 mesh natural flake graphite, 12gKOH, 3gKNO 3 , put into a reaction kettle, add 25ml of deionized water, and stir for 10min. After the reactor was sealed, the gas in the reactor was replaced with oxygen three times, and then filled with oxygen to 5Mpa, the reactor was heated to 450°C, and the reaction was stirred for 36 hours. After the reaction was completed, cool to room temperature, and the reactant was separated from solid and liquid. The solid product was first washed with 5% dilute hydrochloric acid, then washed with deionized water until neutral, and dried at 100°C to obtain 2.752 g of graphene oxide solid. The liquid phase is recovered, and after the concentration of the solution alkali is adjusted according to the concentration of the KOH solution before the reaction, it is recy...
Embodiment 2
[0020] This embodiment illustrates that the graphite is 325 meshes of natural flake graphite, the alkali is KOH, and the pro-oxidant is KNO 3 reaction process and results.
[0021] With 13mL of lye recovered in Example 1, add 6.25gKOH and 17mL deionized water to adjust to the same concentration according to the concentration of KOH measured before the reactor of Example 1 was sealed, and get 3g325 mesh natural flake graphite, 3gKNO 3 , put it into the reaction kettle, add 25ml circulating lye, and stir for 10min. After the reactor was sealed, the gas in the reactor was replaced with oxygen three times, and then the reactor was heated to 600° C. with 3 Mpa of oxygen, and the reaction was stirred for 36 hours. After the reaction was completed, it was cooled to room temperature, the reactant was separated from solid and liquid, and the solid product was washed with 5% dilute hydrochloric acid, then washed with deionized water until neutral, and dried at 100°C to obtain 2.804 g o...
Embodiment 3
[0023] This embodiment illustrates that the graphite is 325 meshes of natural flake graphite, the alkali is NaOH, and the pro-oxidant is NaNO 3 reaction process and results.
[0024] Take 5g325 mesh natural flake graphite, 12gNaOH, 3gNaNO 3 , put into a reaction kettle, add 25ml of deionized water, and stir for 10min. After the reactor was sealed, the gas in the reactor was replaced with oxygen three times, and then the reactor was heated to 450° C. with 5 Mpa of oxygen, and the reaction time was stirred for 36 hours. After the reaction was completed, cool to room temperature, the reactants were separated into solid and liquid, and the solid was washed with 10% dilute hydrochloric acid, then washed with deionized water until neutral, and dried at 100°C to obtain 4.763 g of graphene oxide solid. The liquid phase is recovered, and after the concentration of the solution alkali is adjusted according to the concentration of the NaOH solution before the reaction, it is recycled a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com