Preparation method of antineoplastic drug carrier having dual-lymphatic targeting
An anti-tumor drug and lymphatic targeting technology, which is applied in the direction of anti-tumor drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve high targeting rate, stable physical and chemical properties, and high drug loading rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
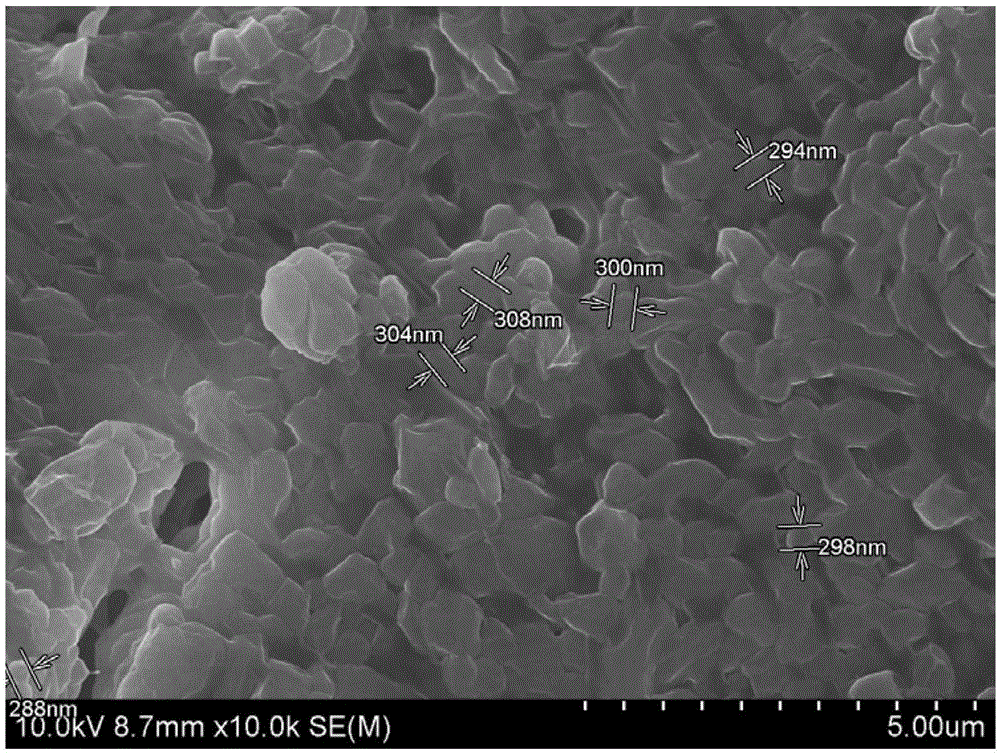
[0034] a. Add 8 parts of chitosan to 4 parts of 1% acetic acid solution and stir for 1 hour. After the chitosan is completely dissolved, pass the solution through 0.45 μm and 0.22 μm filter papers in turn.
[0035] b. Add 5 parts of sodium tripolyphosphate to 4 parts of deionized water, and stir for 30 minutes. After the sodium tripolyphosphate is completely dissolved, pass the solution through 0.45 μm and 0.22 μm filter papers in turn.
[0036] c. Add 4 parts of paclitaxel to 5 parts of methanol and stir for 15 minutes until the drug is completely dissolved.
[0037] d. Add 5 parts of paclitaxel methanol solution dropwise to 5 parts of chitosan solution, and then slowly drop 2 parts of sodium tripolyphosphate solution into the solution under continuous magnetic stirring, and stir for 30 minutes to form a paclitaxel-loaded shell Glycan nanoparticles. Then centrifuge at 12000 rev / min, and freeze-dry the extract in a lyophilizer to obtain paclitaxel-loaded chitosan nanoparticle...
Embodiment 2
[0041] a. Add 10 parts of chitosan to 5 parts of 1% acetic acid solution and stir for 1.5 hours. After the chitosan is completely dissolved, pass the solution through 0.45 μm and 0.22 μm filter papers in turn.
[0042] b. Add 4 parts of sodium tripolyphosphate to 4 parts of deionized water, and stir for 30 minutes. After the sodium tripolyphosphate is completely dissolved, pass the solution through 0.45 μm and 0.22 μm filter papers in turn.
[0043] c. Add 4 parts of paclitaxel to 4 parts of methanol solution and stir for 10 minutes until the drug is completely dissolved.
[0044] d. Add 5 parts of paclitaxel solution dropwise to 5 parts of chitosan solution, under continuous magnetic stirring, slowly add 2 parts of sodium tripolyphosphate solution into the solution, and stir for 30 minutes to form paclitaxel-loaded chitosan Nanoparticles. Then centrifuge at 11000 rev / min, and freeze-dry the extract in a lyophilizer to obtain paclitaxel-loaded chitosan nanoparticle powder.
...
Embodiment 3
[0048] a. Add 9 parts of chitosan to 5 parts of 1% propionic acid solution, stir for 1 hour, after the chitosan is completely dissolved, pass the solution through 0.45 μm and 0.22 μm filter papers successively.
[0049] b. Add 5 parts of sodium tripolyphosphate to 4 parts of deionized water, and stir for 30 minutes. After the sodium tripolyphosphate is completely dissolved, pass the solution through 0.45 μm and 0.22 μm filter papers in turn.
[0050] c. Add 5 parts of doxorubicin to 4 parts of ethanol solution and stir for 20 minutes until the drug is completely dissolved.
[0051] d. Add 6 parts of doxorubicin solution dropwise to 5 parts of chitosan solution, and then slowly add 2 parts of sodium tripolyphosphate solution into the solution under continuous magnetic stirring, and stir for 30 minutes to form loaded doxorubicin chitosan nanoparticles. Then centrifuge at 10,000 revs / min, and freeze-dry the extract in a lyophilizer to obtain chitosan nanoparticle powder of doxor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com