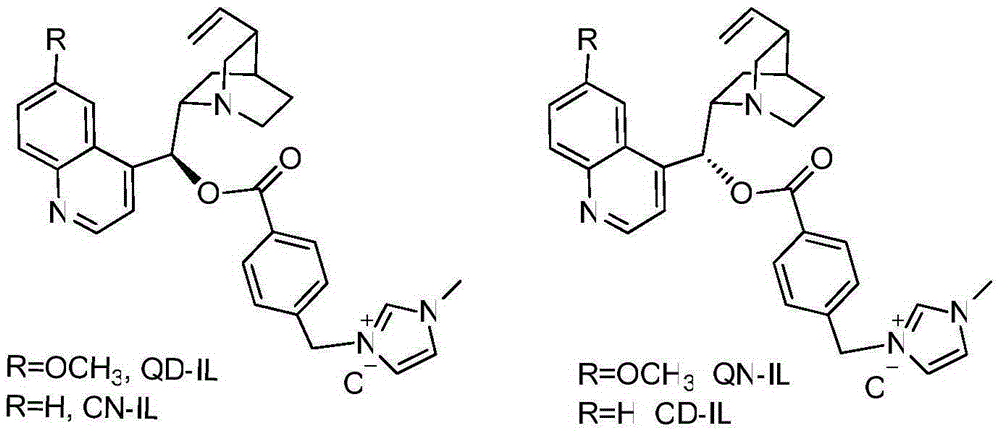
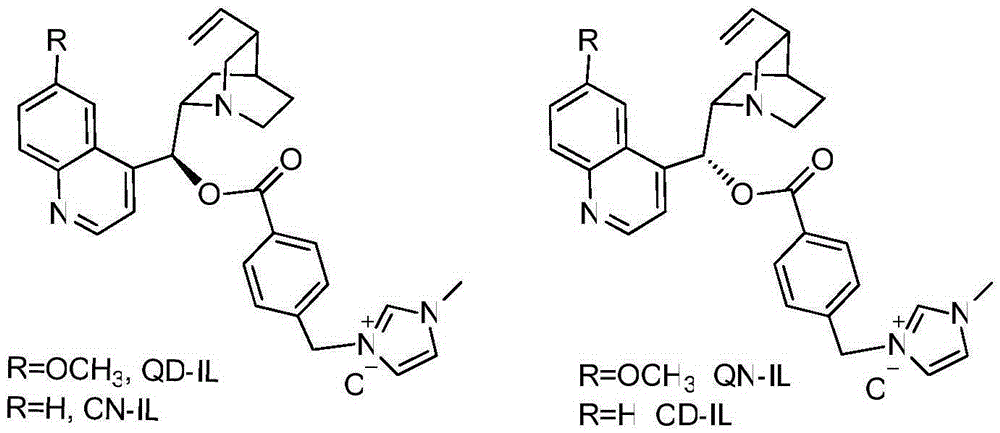
Preparation of imidazole type ionic liquid derived by cinchona alkaloid
An ionic liquid, cinchona base technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, organic chemistry and other directions, can solve the problem of unreasonable utilization of resources, large amount of catalyst, and inability to recycle and other problems, to achieve the effects of easy separation, recovery and recycling, high raw material utilization, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The first step: Weigh 8.68g of Quinine and 2.98g of triethylamine and dissolve them in 40ml of tetrahydrofuran. Dissolve 5.02g of p-chloromethylbenzoyl chloride in 5ml of tetrahydrofuran. The tetrahydrofuran solution was slowly dropped dropwise into the tetrahydrofuran solution of quinine triethylamine. After the dropwise addition, the reaction was carried out at normal temperature and normal pressure for 12 hours. After the reaction was monitored by TLC, the solvent was spin-dried. Mixed and extracted, the aqueous phase was extracted twice with 40 ml of dichloromethane, the organic phases were combined and extracted once with 30 ml of saturated NaCl solution, and an appropriate amount of anhydrous Na was added. 2 SO 4 Drying, then filtering, concentrating the filtrate and silica gel column chromatography (EA:PE=1:5) to obtain the 9-hydroxyl-modified compound of cinchonadine as benzyl chloroalkane (QN-Cl) (3-(4-( ((R)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinu...
Embodiment 2
[0017] The first step: Weigh 4.15g of Quinidine (Quinidine) and 1.53g of triethylamine and dissolve them in 20ml of acetonitrile, dissolve 2.46g of p-chloromethylbenzoyl chloride in 3ml of tetrahydrofuran, and at 0 ℃ temperature, The tetrahydrofuran solution of the acid chloride was slowly dropped dropwise into the acetonitrile solution of quinidine triethylamine. After the dropwise addition, the reaction was carried out at normal temperature and normal pressure for 16 hours. After the reaction was monitored by TLC, the solvent was spin-dried. Mixed and extracted with equal volume of water, the aqueous phase was extracted twice with 40 ml of dichloromethane, the organic phases were combined and extracted once with 30 ml of saturated NaCl solution, and an appropriate amount of anhydrous Na was added. 2 SO 4 Dry, then filter, concentrate the filtrate and silica gel column chromatography (EA:PE=1:3) to obtain the compound as benzyl chloroalkane (QD-Cl) (3-(4-(((S)-(6-methoxyquino...
Embodiment 3
[0021] The first step: Weigh 8.59g of Cinchonine and 3.24g of triethylamine and dissolve them in 50ml of dimethylformamide (DMF). Under the temperature of ℃, the tetrahydrofuran solution of the acid chloride was slowly dropped dropwise into the DMF solution of quinidine triethylamine, after the dropwise addition, the reaction was carried out at normal temperature and normal pressure for 16 hours, and the solvent was spin-dried after the reaction was monitored by TLC. Ethyl acetate, diluted with 200ml of distilled water, the precipitated white solid was collected by filtration, the filtrate was re-separated, the aqueous phase was extracted twice with 40ml of ethyl acetate, the combined organic phases were extracted once with 20ml of saturated NaCl solution, and an appropriate amount of anhydrous Na 2 SO 4 Dry, then filter, concentrate the filtrate and silica gel column chromatography (EA:PE=1:5) to obtain the compound as benzyl chloride (CN-Cl)(1-methyl-3-(4-(((S)- quinolin-4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com